Translate this page into:
Comparison of fresh versus frozen embryo transfer in women with polycystic ovary syndrome
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Transfer of fresh embryos is a usual practice but in women at risk of ovarian hyperstimulation due to excess follicle development, elective cryopreservation of all embryos followed by transfer in subsequent cycle is preferred. Fresh cycles have supraphysiological steroid levels which may alter the endometrial receptivity and probably affect placentation adversely whereas frozen embryo transfer is performed to the uterus after a programmed physiologic cycle of hormone replacement to prepare the endometrium.
Objective:
To find out whether frozen embryo transfer in subsequent cycle is better than that in fresh transfer in women with polycystic ovary syndrome (PCOS) when human chorionic gonadotropin (hCG) is used as a trigger in antagonist cycles.
Study Design, Size, Duration:
It is a prospective cohort study. Infertile women <35 years with the PCOS diagnosed by Rotterdam criteria who were undergoing their first in vitroPlease check whether the suggested full form for the acronyms in vitro fertilization (IVF) and assisted reproductive techniques (ART) provided in the article is correct. fertilization cycle from 1st January 2015 to 28th February 2016 were included. Cycles complicated by ovarian hyperstimulation syndrome were excluded.
Participants/Materials, Setting, Methods:
Women (N = 126) with terminal estradiol levels below 2500 pg/ml were triggered with recombinant hCG, and based on the number of retrieved oocytes, they were divided into two groups: Group A <15 oocytes retrieved had fresh embryo transfer on day 3 and Group B where >15 oocytes were retrieved, but all embryos were frozen on day 3 and transferred in subsequent cycle. Primary outcomes were clinical pregnancy rates and live birth rates. Secondary outcomes were fertilization, implantation and miscarriage rates.
Results:
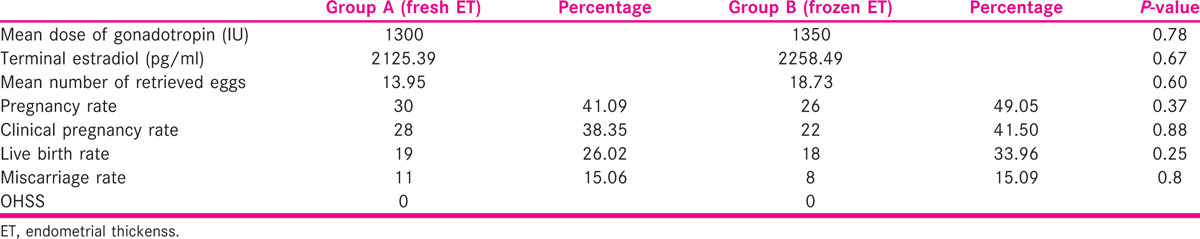
Group A had 73 fresh transfer, and Group B had 53 frozen embryo transfer. Both groups were comparable regarding age, body mass index, basal follicular stimulating hormone, antimullerian hormone and antral follicle count. Categorical data were represented as frequency and percentages, differences in these measures between the groups were compared using chi-square tests, and quantitative data were analyzed by using student t test. Clinical pregnancy rates (Group A: 38.4% versus Group B: 41.5%, P = 0.88) and live birth rate (Group A: 26.0% versus Group B: 33.9%, P = 0.25) were slightly higher in Group B though not statistically significant. The miscarriage rate in both the groups was comparable (Group A: 15.1% and Group B: 15.1%, P = 0.8).
Keywords
Frozen embryo transfer (FET) in PCOS
fresh versus frozen embryo transfer
OHSS
terminal estradiol level
INTRODUCTION
In vitro fertilization (IVF) is a commonly performed assisted reproductive technology procedure. There are concerns about the maternal and fetal safety of the procedure. Ovarian hyperstimulation syndrome (OHSS) is a life-threatening complication. Prevention of OHSS in high-risk populations has become a major goal in the research of controlled ovarian stimulation (COS) in IVF programs. Withholding human chorionic gonadotropin (hCG) and cycle cancellation is considered the safest option but carries adverse emotional and financial implications. Withholding treatment for several days (coasting) has been shown to diminish the incidence and severity of OHSS without compromising pregnancy rates.[1] The introduction of GnRH agonist trigger does prevent OHSS but compromises on pregnancy rates due to accelerated leuteolysis, which can be taken care of with dual trigger.[2,3]
Frozen embryo transfer is thought to give better results as compared to fresh transfer probably due to disturbed receptivity due to elevated steroidal levels in stimulated cycles.[4] In polycystic ovary syndrome (PCOS) females the endometrial receptivity is also disturbed due to reduced expression of αvβ3 integrin and glycodelin.[5] Observational studies have shown that rates of singleton pregnancy are higher after frozen embryo transfer than after fresh embryo transfer.[6] A meta-analysis of 11 observational studies showed that pregnancies from frozen embryo transfer were associated with lower risks of preterm birth, low birth weight and perinatal death than were pregnancies from fresh embryo transfer.[7] Subsequently, small, single-center, randomized studies comparing the two procedures have shown better ongoing pregnancy rates with frozen embryo transfer.[8] However, data are lacking from randomized trials to determine any differences between the two procedures in live birth rates and pregnancy complications.
PARTICIPANTS AND METHODS
Study design, size, duration
This study was performed at Akanksha IVF centre. Women with the PCOS who were undergoing their first IVF cycle from 1st January 2015 to 28th February 2016 were eligible.
To diagnose the PCOS, we used modified Rotterdam criteria,[9] which included menstrual abnormalities (irregular uterine bleeding, oligomenorrhea or amenorrhea) combined with either hyperandrogenism or polycystic ovaries. Hyperandrogenism was diagnosed on the basis of either hirsutism or hyperandrogenemia. Hirsutism was determined by means of a modified Ferriman–Gallwey score of more than 6 (on a scale of 0–36, with higher scores indicating increased hair growth) at the screening examination.[10] Hyperandrogenemia was defined as an elevated total testosterone level according to local laboratory criteria. Polycystic ovaries were defined as the presence of either an ovary containing 12 or more antral follicles measuring 2–9 mm in diameter or an increased ovarian volume (>10 cm3). Other causes of hyperandrogenism and ovulation dysfunction — including tumors, congenital adrenal hyperplasia, hyperprolactinemia and thyroid dysfunction were ruled out. All the patients received a standardized ovarian stimulation regimen, oocyte retrieval and fertilization, followed by a planned transfer of up to two day-3 embryos. In brief, recombinant follicle-stimulating hormone (Follisurge, Intas) at a daily dose of 150 IU for patients weighing 60 kg or less and 187.5 IU for those weighing more than 60 kg was started on day 2 or 3 of the menstrual cycle. These doses were adjusted according to the ovarian response, as monitored on ultrasonography and the measurement of serum sex steroids. Gonadotropin-releasing hormone antagonist (Cetrolix, Intas) at a daily dose of 250 μg was started on day 6 of gonadotropin. Serum estradiol levels were measured on the day of trigger, if less than 2500 pg/ml recombinant hCG (Ovitrelle, Merck Serono) at a dose of 250 μg (6500 IU), and GnRH agonist was administered to induce oocyte maturation when serum estradiol level was more than 2500 pg/ml. Only women with recombinant human chorionic gonadotropin trigger were included in the study. Oocyte retrieval was performed 34–36 h later. On the day of oocyte retrieval, based on the number of retrieved participants, they were divided into two groups: Group A <15 oocytes retrieved had fresh embryo transfer on day 3 and Group B where >15 oocytes were retrieved, all embryos were frozen on day 3, and transferred in subsequent cycle.
For patients who were assigned to the fresh-embryo group, micronized progesterone pessary at a daily dose of 400 mg twice a day vaginally was started for luteal-phase support, beginning on the day of oocyte retrieval until 10 weeks after conception. For patients who were assigned to the frozen-embryo group, no luteal-phase support was administered after oocyte retrieval, and day-3 embryos were cryopreserved for later transfer. Oral estradiol valerate (Progynova) was administered for endometrial preparation on day 2 or 3 of the subsequent menstrual cycle after oocyte retrieval. Micronized progesterone pessary at a daily dose of 400 mg twice a day vaginally was added when the endometrial thickness reached 7 mm or more. After 4 days of the progesterone regimen, two day-3 frozen embryos were thawed and transferred. The luteal-phase support with estradiol valerate and micronized progesterone pessary for endometrium preparation continued until 10 weeks after conception. Cycles complicated by OHSS were excluded.
Embryo culture, evaluation and selection for transfer
The oocytes were inseminated approximately 4–6 h after follicular aspiration by a conventional method or intracytoplasmic sperm injection, according to the sperm quality. Morphologic criteria were used for embryo scoring. On day 3, two high-quality embryos were picked out for fresh transfer or cryopreserved by means of vitrification in the group undergoing frozen embryo transfer. Biochemical pregnancy was defined as a human chorionic gonadotropin level of more than 10 mIU per milliliter, as measured at 14 days after embryo transfer. Clinical pregnancy was defined as the presence of a gestational sac in the uterine cavity at 35 days after embryo transfer, as detected on ultrasonography. Ongoing pregnancy was defined as the presence of a fetus with heart motion at 11–12 weeks of gestation. All pregnancy and neonatal outcomes were obtained through a review of medical records. Moderate and severe OHSSs were defined according to accepted criteria.
Study outcomes
Primary outcomes were clinical pregnancy rates and live birth rates. Secondary outcome were miscarriage rate and OHSS incidence.
Statistical analysis
Categorical data were represented as frequency and percentage; differences in these measures between the study groups were assessed by means of chi-square analysis, with the use of Fisher’s exact test for expected frequencies of less than. Continuous data were expressed as means (±SD, standard deviation), with a Wilcoxon rank-sum test for between-group differences. The statistical analysis was performed using Statistical Package for the Social Sciences software (SPSS Inc., Chicago, IL, United States).
RESULTS
Study patients
The baseline characteristics of the patients were similar in the two study groups [Table 1].

Group A had 73 fresh transfer and Group B had 53 frozen embryo transfer. Both groups were comparable regarding age, body mass index (BMI), basal follicular stimulating hormone, antimullerian hormone (AMH) and antral follicle count. Categorical data were represented as frequency and percentages, differences in these measures between the groups were compared using chi-square tests and quantitative data by student t test. Clinical pregnancy rates (Group A: 38.4% versus Group B: 41.5%, P = 0.88), live birth rate (Group A: 26.0% versus Group B: 33.9%, P = 0.25) were slightly higher in Group B though not statistically significant. The respective miscarriage rates in both the groups were comparable (Group A: 15.1% and Group B: 15.1%, P = 0.8) [Table 2].
DISCUSSION
There is a continuous strive to improve pregnancy outcomes in IVF cycle. Also in women with PCOS IVF cycle is sometimes segmented to avoid the complication of OHSS. The introduction of GnRH agonist trigger followed by freeze all has almost completely eliminated the risk of OHSS. It is seen that with hCG trigger and comparable steroid levels the yield of oocytes can be different. At an average estradiol of 2258.49 pg/ml 18.73 oocytes were retrieved in Group B. It is known that elevated estradiol levels affect the endometrial receptivity. More number of oocytes retrieved in PCOS women with average estradiol levels may affect the receptivity negatively more than when a lesser number of oocytes are retrieved. In absence of any definitive biochemical tests, the decision whether to go ahead with fresh transfer or freeze all is on the discretion of the consultant. Here in, we found out that with average estradiol levels (<2500 pg/ml) the number of retrieved oocytes guided to freeze all and transfer in subsequent cycle, yielding better pregnancy and live birth rates even in hCG-triggered cycles.
Wider implications of the findings
Performing frozen embryo transfer in PCOS women with average estradiol levels yet higher number of recovered oocytes is better than fresh transfer, implying adopting practice of routine elective freezing of embryos and transfer in subsequent cycle for better reproductive outcomes even in hCG-triggered cycles in PCOS women.
Limitations, reasons for caution
Due to the small sample size, the study lacks power, which is the main limitation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Coasting for the prevention of ovarian hyperstimulation syndrome: much ado about nothing? Fertil Steril. 2006;85:547-54.
- [Google Scholar]
- Gonadotropin-releasing hormone agonist to induce final oocyte maturation prevents the development of ovarian hyperstimulation syndrome in high-risk patients and leads to improved clinical outcomes compared with coasting. Fertil Steril. 2010;94:1111-4.
- [Google Scholar]
- GnRH agonist for triggering of final oocyte maturation: Time for a change of practice? Hum Reprod Update. 2011;17:510-24.
- [Google Scholar]
- Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: A systematic review and meta-analysis. Fertil Steril. 2013;99:156-62.
- [Google Scholar]
- The endometrium in polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes. 2002;9:480-5.
- [Google Scholar]
- Perinatal morbidity after in vitro fertilization is lower with frozen embryo transfer. Fertil Steril. 2011;95:548-53.
- [Google Scholar]
- Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: A systematic review and meta-analysis. Fertil Steril. 2012;98:368-77.
- [Google Scholar]
- Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: A prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96:344-8.
- [Google Scholar]
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41-7.
- [Google Scholar]
- Clinical assessment of body hair growth in women. J Clin Endocrinol Metabol. 1961;21:1440-7.
- [Google Scholar]







