Translate this page into:
Tracking the implantation window: Synchronizing endometrial preparedness for implantation with stage of blastocyst to be transferred in antagonist IVF cycles involving single blastocyst transfers
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction
The day of menstrual period is usually not taken into account during embryo transfer in IVF cycles.
Aim
We sought to track the relevance of ‘implantation window’ by contemplating a correlation between stage of blastocyst transferred and endometrial preparedness for implantation with respect to day of menstrual period in antagonist in vitro fertilization (IVF) cycles.
Design, Materials and Methods
This study involved retrospective analysis of 443 cycles in women undergoing antagonist treatment protocol followed by oocyte-retrieval approximately between days 12 and 16 (rarely on days 17 and 18 in case of long follicular phase) of their menstrual period. All cycles involved day 5/6 single blastocyst-transfer (sBT) of top (AA) or good (AB/BA) quality blastocysts of various stages. Slightly modified Gardner’s system for blastocyst-stage grading was followed. Inner cell mass, trophectoderm, were graded as A, B, C as per Gardner’s system. Clinical pregnancy rate (CPR) and live birth rate (LBR) were main outcome measures.
Results
Overall CPR = 26.64% (118/443), whereas LBR was 21.67% (96/443). CPR was influenced by transfer of various stages (1–6) of the blastocysts on different days of menstrual period (days 17–24, covering the implantation window). Our results indicate that as day of menstrual period advances during the window period, higher stage blastocyst-transfer enhances the odds of a live birth.
Conclusion
Synchrony between stage of blastocyst transferred and the endometrial preparedness for implantation with respect to day of menstrual cycle has a definitive influence on LBR in IVF cycles. Asynchrony and out of phase BT may lead to missing out the implantation window and unnecessarily hamper CPR/LBR.
Keywords
Blastocyst
endometrium
implantation window
IVF
live birth
INTRODUCTION
In nature, efficient reproduction relies on the synchronized behaviour of animals, the synchronized physiology of their reproductive organs and the synchronized interaction of the male and female gametes. This fundamental principle of synchronization has to be respected in assisted reproductive technology (ART) irrespective of the technique or species involved. Asynchrony and out of phase embryo transfer may lead to missing out the implantation window and unnecessarily hamper pregnancy rates.
It has been reported that increased uterine contractility during early luteal phase may adversely affect implantation potential of day 3 cleavage stage embryos.[1,2] Conversely, the decline in this contractility towards the late luteal phase may enhance the probability of embryo implantation.[3] This can simply be achieved by subjecting embryos to extended culture till day 5/6 post ovum pickup (OPU). With more stringent quality control measures in place and with the advent of better culture conditions using sequential media, day 5/6 blastocyst transfer (BT) indeed seemed to be a beneficial proposition for increased pregnancy rates. Several arguments have been offered in favour of extended culture.[4] The most relevant being the observation that whereas not all top quality cleavage stage embryos form viable blastocysts, morphometric selection of the best looking day 5 blastocysts has considerably lowered aneuploidy rates. Blastocyst culture also enables transfer of fewer number of embryos per cycle and gives a better choice to opt for elective single embryo transfer (eSET), consequently resulting in reduced multiple gestation rates. An added advantage of blastocyst culture is that it offers adequate duration for gonadotrophin-induced raise in supraphysiological E2 levels to subside, thereby also providing ample time for improvement in endometrial receptivity (better temporal synchronization between embryo and endometrium during transfer) and eventually higher implantation rates.[4]
Consequently, there were several studies suggesting not just BT but eSET to increase implantation rates.[5,6,7] The trend of transferring expanded blastocysts continued till newer studies prompted assisted hatching, and yet another study[8] reported better implantation rates with spontaneously hatching/hatched blastocysts. However, contradictory reports have kept pouring in. Whereas randomized trials in good prognosis patients revealed better implantation rates with BT compared to cleavage stage embryo transfer,[9] subsequent trials in unselected patients revealed conflicting results. In addition, in unselected patients with previous one or more failed cycles, there was no difference in pregnancy and live birth rates (LBRs) with day 5 blastocyst or day 3 embryo transfer.[10,11] Recent studies have also reported that BT does not improve the likelihood of pregnancy in poor prognosis patients.[9] Attempts were then focused towards identification of ‘window’ period of implantation. ‘Implantation window’ is defined as the stipulated time during which the endometrium is rendered receptive for incoming embryo and outside of which time frame, it becomes non-receptive for implantation. Despite several studies focusing on the two most confounding factors, embryo development and endometrial receptivity, no substantial increase in take-home baby rates has been observed. The intricate mechanism of implantation still remains an enigma and ‘increased LBRs following in vitro fertilization (IVF)’ has been an Achilles’ heel for embryologists and clinicians alike!
We therefore tried to follow the basic premise that understanding the intricate correlation between embryo development and implantation window may be the key towards comprehending the implantation orchestra. The implantation window is generally believed to encompass days 17 to 24 of the menstrual cycle, during which period embryonic development and endometrial preparation for implantation occur in tandem. An embryo undergoes different stages of blastocyst development before it implants into a receptive endometrium. We contended that even a ‘top’-quality blastocyst transferred within the window period may fail to implant in an ultrasonographically detected healthy endometrium if it is not at the right stage of development on that particular day of period when transfer was performed. Therefore, our study sought to track the ‘implantation window’ by contemplating a relevant correlation between stage of blastocyst transferred and endometrial preparedness for implantation with respect to day of menstrual period in antagonist IVF cycles.
MATERIALS AND METHODS
Retrospective analysis of 443 cycles (February 2012 to September 2014) in eumenorrhic women with no previous implantation, undergoing IVF with antagonist treatment protocol using recombinant follicle stimulating hormone (FSH) for ovarian stimulation (Recagon 200 IU daily from day 2 to day of human chorionic gonadotropin (hCG) trigger). Regular ultrasonographic monitoring of the follicles was performed. When ≥2 follicles reached a size of 18-mm diameter, final trigger for oocyte maturation (injection hCG 5000 IU) was given. Oocyte retrieval was performed between 34 and 36 h after hCG trigger. Luteal phase support in the form of the micronized progesterone (Susten 100 mg daily) was provided. Oocyte/embryo donation and intra-cytoplasmic sperm injection (ICSI) cycles as well as freeze–thaw/vitrified–warmed embryo transfers were excluded to obviate any biases and to obtain a homogenous population of the women for study.
Embryo culture
Embryo culture was performed using Cooks sequential media as described in our earlier study.[8] Briefly, embryos were cultured in individual microdrops of 20 μl: from day 0 to 1 in fertilization media, days 1 to 3 in cleavage media and days 3 to 5/6 in blastocyst media and incubated at 37°C in bench-top incubator with a triple gas mixture (6% CO2, 5% O2 and 89% N2). Embryonic development was monitored daily and micro-photographic images obtained at a specific time till day of transfer. As per our routine laboratory practice, three embryologists (one senior and two junior assistants) coordinated the entire process of embryo culture, development and assessment to ensure implementation of a standardized protocol and remove any observer subjectivity in embryo assessment. Gardner and Schoolcraft’s blastocyst grading system[12] was applied as it was the most widely used method during the course of our study. However, a later, retrospective application of the nurture simplified blastocyst grading system[13] to saved images of transferred blastocysts also substantiated our selection method because there was intra-observer consensus between the two methods regarding grade of the blastocyst that had been selected for transfer. Cycles involving day 3 cleavage stage embryo transfer and also those involving transfer of two or three day 5/6 blastocysts were excluded from this analysis. Therefore, this study primarily involved day 5/6 single BT (sBT) of the top (AA) or good (AB/BA) quality blastocysts (BC) of various stages.
Blastocyst grading
Gardner’s system for blastocyst stage grading[12] was followed, albeit with minor modification [Figure 1]:
Stage 1: Early blastocyst − blastocele <50% volume of the embryo
Stage 2: Full blastocyst − blastocele completely fills the embryo
Stage 3: Expanded blastocyst − blastocele volume larger than that of early embryo accompanied by thinning of zona pellucida (ZP)
Stage 4: Point of hatching blastocyst − trophectoderm starts herniating through ZP
Stage 5: Hatching blastocyst: blastocyst has emerged out >25% from zona almost giving appearance of figure ‘8’
Stage 6: Hatched blastocyst: blastocyst completely escapes from zona.

- Blastocysts: different stages and grades. Number represents stage of blastocyst, the first alphabet = grade of inner cell mass (ICM), second alphabet = grade of trophetodermal (TE) cells
For blastocysts graded as 3 to 6, development of inner cell mass was assessed as follows:
tightly packed, many cells,
loosely grouped, several cells and
very few cells.
The trophectoderm was assessed as follows:
many cells forming a cohesive epithelium,
few cells forming a loose epithelium or
very few large cells.
Endometrial development
Endometrium was monitored ultrasonographically and assessed as described by Sher et al.[14] Briefly, on day of sBT, endometrial thickness was measured and graded according to the endometrial pattern (multi-layered or a non-multilayered) visualized using transvaginal sonography. Endometrial thickness (centimetres) was derived from average measurements in each plane (longitudinal and transverse) of the distance between the endometrial and myometrial interfaces at the level of the uterine fundus. Endometrial layers (pattern) reflected ultrasonologically discernible layers in the endometrium, ranging from one to four.[14] sBT was performed on day 5/6 of OPU only if the endometrial thickness was ≥0.8 cm (but not more than 1.3 cm) and if it was grade IV. No adjuvant therapy in the form of endometrial scratching, granulocyte-colony stimulating factor (G-CSF) perfusion or low molecular weight heparin etc. was provided to any of the women. Luteal phase support with micronized progesterone was provided to all women.
Outcome measures and analysis
Serum βhCG measurement on day 14 post transfer indicated pregnancy. Presence of the gestational sac with positive cardiac activity by sixth week of gestation confirmed clinical pregnancy. Supernumery blastocysts after transfer, whether top or average quality, were frozen. LBR was the main outcome measure. Statistical analysis was performed using Graph Pad Prism V software (GraphPad Software, Inc., La Jolla, California, USA) to obtain statistical significance [one-way analysis of variance (ANOVA), odds ratio]. A P-value of <0.05 was considered significant. Because this is a retrospective study, it was felt redundant to calculate power of the study.
RESULTS
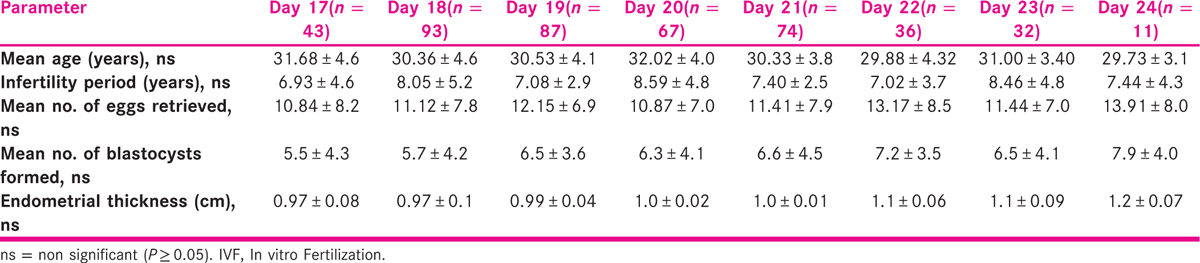
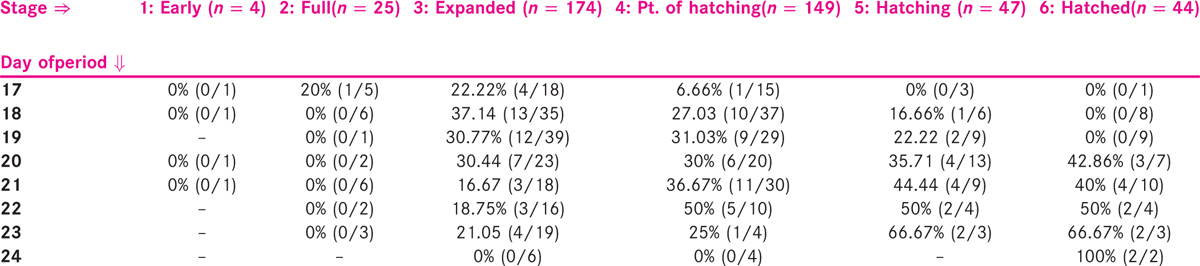
Overall clinical pregnancy rate (CPR) was found to be 26.64% (118/443), whereas LBR was 21.67% (96/443). No cases of monozygotic twinning were observed. There was no statistically significant difference between the study group in the various IVF parameters [Table 1], suggesting that it was an unbiased homogenous study population. The levels of E2 (2097 vs. 2041 vs. 2316 vs. 2128 vs. 2140 vs. 2149 vs. 2093 vs. 2158 pg/ml; one-way ANOVA P = 0.75), luteinizing hormone (LH) (3.5 vs. 3.0 vs. 3.0 vs. 3.7 vs. 2.7 vs. 3.9 vs. 2.7 vs. 2.4 IU/l; one-way ANOVA P = 0.05) and progesterone (1.14 vs. 1.32 vs. 1.4 vs. 1.19 vs. 1.31 vs. 1.28 vs. 1.22 vs. 1.3 ng/ml; one-way ANOVA P = 0.57) on day of hCG also did not differ significantly among the groups based on transfers on day 17 through day 24, respectively. Similarly, the luteal phase levels of E2 (1074 vs. 927 vs. 1018 vs. 751 vs. 783 vs. 1057 vs. 755 vs. 631 pg/ml; one-way ANOVA P = 0.21) and progesterone (62 vs. 67 vs. 55 vs. 65 vs. 52 vs. 54 vs. 57 vs. 49 ng/ml; one-way ANOVA P = 0.11) on day 14 post sBT did not show any significant differences among the various groups. However, retrospective analysis revealed that LBR was influenced by transfer of various stages (1–6) of the blastocysts on different days of menstrual period (days 17–24, covering the implantation window) as depicted in Table 2. The likelihood of live birth is greatly enhanced with the transfer of the full blastocyst on day 17 (odds ratio: 11.18), Expanded blastocyst on days 18 to 20 (odds ratio: 1.8), blastocyst with zona breaker cells/point of hatching on days 21, 22 (odds ratio: 1.6) and hatching/fully hatched blastocyst on days 23, 24 (odds ratio: 2.83) compared to their transfers on any other day of the menstrual cycle. In our study, no clinical pregnancies or live births were obtained with the transfer of the early stage blastocyst on any of the days of the menstrual period. Expanded stage blastocysts on days 18 to 20; point of hatching blastocysts on days 21, 22; and hatching/hatched stage blastocysts on days 23, 24 correlated more strongly with live birth (Pearson r = 0.31, 0.41 and 0.75, respectively) than transfer of these stages of the blastocysts on any other days encompassing the implantation window period.
DISCUSSION
Our results raise a pertinent question whether it is absolutely irrelevant to take into account the day of menstrual period while doing embryo transfer in IVF cycles? Debating the existence of the implantation window (∼days 18–22 of natural cycle) or considering day of the menstrual period may not hold much significance in IVF cycles involving day 3 cleavage stage embryo transfers. However, with an excellent blastocyst gradation system available and a rising trend towards day 5/6 BTs, it seems absolutely valid to investigate whether stage of the blastocyst transferred has any influence on CPRs if this transfer is synchronized with the day of the menstrual period. In addition, the gonadotrophin-induced raise in supraphysiological E2 levels is known to subside due to the extended culture.[15] Most importantly, the temporal synchronization between embryo and endometrium almost mimics the physiological state owing to embryo culture to blastocyst stage.[16,17]
This study provides a non-invasive insight into the so-called mythical implantation window and endometrial preparedness for implantation. It may be justified to mention that the implantation window can be rendered redundant and a mythical entity if the stage of the blastocyst transferred during this period is not taken into consideration. This study amply justifies the differential endometrial response to implantation observed during the implantation window period vis-à-vis stage of the blastocyst transferred. To our knowledge, no study has ever tried to correlate the days of the menstrual cycle during implantation window period with the stage of the blastocyst transferred.
In an attempt to enhance the overall take-home baby rates, recent efforts have focused on identifying clinical factors associated with blastocyst development and pregnancy. It has been believed that a non-multilayered endometrium consisting of homogeneous endometrial pattern (i.e. grade 4/IV) characterized by either hyper-echogenic or iso-echogenic endometrium is indicative of favourable endometrial response.[18] However, because luteal phase support in the form of micronized progesterone was provided to all women and we transferred blastocysts only when endometrial thickness and echo pattern were in the favourable range, this ultrasonographic parameter seems redundant and further vindicates our point that the stage of the blastocyst probably triggers some endocrinological events in the endometrium which is of utmost importance for better receptivity and higher pregnancy rates. Latest research also proposes embryo glue[19] and endometrial scratch[20,21] as adjunct endometrial factors that may facilitate increased receptivity. However, trials are still underway and no conclusive evidence is yet available for the benefits of these techniques in improving LBRs in IVF cycles. Some studies have also suggested a role for serum 25-hydroxy-vitamin D levels in predicting implantation and CPRs,[22] whereas its levels in follicular fluid have been reported to lower the possibility of achieving pregnancy.[23] It would therefore be interesting to carry out a study combining any of these endometrial factors along with the considerations proposed in our present study.
CONCLUSION
The primary finding of our study is that as the day of the menstrual period advances, higher stage BT gives better pregnancy rates. This study emphasizes that synchrony between stage of the blastocyst transferred and the endometrial preparedness for implantation with respect to day of the menstrual cycle has a definitive influence on pregnancy rates in IVF cycles. Thus, the most practical application of these results is that if embryo development leads or lags with respect to endometrial receptivity, blastocysts may be frozen and transferred in the next conducive, coordinated natural cycle. The results may help establish a new paradigm for synchronized development of the embryo and endometrium for better pregnancy rates in IVF cycles.
Limitation of study
The limitation of our study is that we took into account transfer of only top and good-quality blastocysts in an effort to minimize the influence of embryo quality on pregnancy rates. In addition, it would be better to segregate and compare cycles where all women have undergone oocyte retrieval on similar days of menstrual period so that women can be classified into groups on the basis of same number of days and dose of stimulation. Finally, although we have quite a decent overall sample size, the number of cycles in each group and sub-groups is considerably reduced. Therefore, our results may be considered indicative and larger multi-centric trials are required to reiterate the findings of our study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Hormonal influence on the uterine contractility during controlled ovarian hyperstimulation. Hum Reprod. 2000;15:90-100.
- [Google Scholar]
- Uterine contractions at the time of embryo transfer alter pregnancy rates in in-vitro fertilization transfers. Hum Reprod. 1998;13:1968-74.
- [Google Scholar]
- Uterine contractility decreases at the time of blastocyst transfers. Hum Reprod. 2001;16:1115-9.
- [Google Scholar]
- Blastocyst culture and transfer in clinical-assisted reproduction: A committee opinion. Fertil Steril. 2013;99:667-72.
- [Google Scholar]
- A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod. 1998;13:3434-40.
- [Google Scholar]
- High implantation andpregnancy rates with transfer of human hatching day 6 blastocysts. Fertil Steril. 2001;75:832-3.
- [Google Scholar]
- Comparison of pregnancy outcomes in elective single blastocyst transfer versus double blastocyst transfer stratified by age. Fertil Steril. 2010;93:1837-43.
- [Google Scholar]
- Transfer of spontaneously hatching or hatched blastocyst yields better pregnancy rates than expanded blastocyst transfer. J Hum Reprod Sci. 2013;6:183-8.
- [Google Scholar]
- Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev 2016:CD002118.
- [Google Scholar]
- Live birth rate is significantly higher after blastocyst transfer than after cleavage stage embryo transfer when atleast four embryos are available on day 3 of embryo culture. Hum Reprod. 2005;20:3198-203.
- [Google Scholar]
- In vitro fertilization with single blastocyst stage versus single cleavage stage embryos. N Engl J Med. 2006;354:1139-46.
- [Google Scholar]
- In vitro culture of human blastocysts. In: Jansen R, Mortimer D, eds. Toward reproductive certainty: Fertility and genetics beyond 1999. UK: Parthenon Publishing London; 1999. p. :378-88.
- [Google Scholar]
- A clinically useful simplified blastocyst grading system. Reprod Biomed Online. 2015;31:523-30.
- [Google Scholar]
- Assessment of the late proliferative phase endometrium by ultrasonography in patients undergoing in-vitro fertilization and embryo transfer (IVF/ET) Hum Reprod. 1991;6:232-7.
- [Google Scholar]
- Physiology of implantation. 10th World congress on in vitro fertilization and assisted reproduction, Vancouver, Canada 1997
- [Google Scholar]
- The role of ultrasonography in the evaluation of endometrial receptivity following assisted reproductive treatments: A critical review. Hum Reprod Update. 1996;2:323-35.
- [Google Scholar]
- Adherence compounds in embryo transfer media for assisted reproductive technologies. Cochrane Database Syst Rev (2):CD007421.
- [Google Scholar]
- Endometrial receptivity enhancement through induced injury and repair during ovarian stimulation: The Receptivity Enhancement by Follicular-phase Renewal after Endometrial ScratcHing (REFRESH) trial protocol. Hum Reprod Open. 2017;2017:1-7.
- [Google Scholar]
- Endometrial scratch injury before intrauterine insemination: Is it time to re-evaluate its value? Evidence from a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2018;109:84-96.e4.
- [Google Scholar]
- Effect of vitamin D status on clinical pregnancy rates following in vitro fertilization. CMAJ Open. 2013;1:E77-82.
- [Google Scholar]
- Vitamin D in human reproduction: The more, the better? An evidence-based critical appraisal. Eur Rev Med Pharmacol Sci. 2017;21:4243-51.
- [Google Scholar]







