Translate this page into:
Age-dependent decline of anti-Müllerian hormone (AMH) in a large population of Indian fertile women as measured using the automated VIDAS® AMH assay
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Serum anti-Müllerian hormone (AMH) testing is now routine practice to assess the ovarian follicular reserve and to predict the response to controlled ovarian stimulation (COS) for assisted reproductive technique (ART). Reference values for a new AMH assay should take into account possible genetics and environmental factors that can be observed in a given population/geographical region such as India.
Objectives:
Primary objective was to observe the inter-individual variability of AMH in a large sized cohort of fertile Indian women as measured with the automated VIDAS® AMH assay, to model the age-dependent decline of AMH across the reproductive age range and to establish the reference values for this assay for an Indian female population. Secondary objective was to study the possible relationships between AMH and other parameters such as Vitamin D status and body mass index (BMI).
Materials and methods:
Serum AMH and Vitamin D concentrations were measured using VIDAS® AMH and VIDAS® 25 OH Vitamin D Total assays, in a cohort of 975 fertile Indian women aged 19–50 years. Correlations between AMH and age, Vitamin D, and BMI were also analyzed.
Results:
Reference AMH values for the VIDAS® AMH assay are reported as an AMH/age nomogram, including a model fitting the age-dependent decline of the hormone. For 66.26% of these women, a Vitamin D deficiency was observed.
Discussion and conclusion:
Reference values representative of Indian women are reported using VIDAS® AMH assay that can help the clinicians for the management of their Indian patients consulting for endocrinology and fertility disorders across the reproductive age range. The cross-sectional data analysis of the Vitamin D status has shown a predominant deficiency for these fertile women.
Keywords
Anti-Müllerian hormone
Indian population
nomogram
Vitamin D
INTRODUCTION
In females, anti-Müllerian hormone (AMH) is produced by the granulosa cells of preantral and small antral follicles.[1] Levels of AMH increase during childhood to a peak value around the age of 25 years, followed by a decline to undetectable levels by the age of menopause.[2,3,4] The concentration of AMH in blood is now considered to reflect the size of the ovarian follicular reserve and testing this hormone is routinely performed for various indications such as the assessment of the ovarian reserve to propose optimal options for infertile women regarding assisted reproductive technology (ART), the prediction of response to controlled ovarian stimulation (COS),[5,6,7,8] the follow-up of ovarian function after gonadotoxic treatment,[9,10,11] the endocrine investigation of disorders like polycystic ovary syndrome (PCOS),[12] and possibly the prediction of menopause. Ethnicity has been reported to impact the ovarian reserve via different possible ways including genetics, environmental, geographical, and cultural factors.[13] A previous study comparing Spanish and Indian infertile women who underwent ART has reported that significant differences exist between Spanish and Indian women regarding ovarian reserve markers, with results suggesting a 6-year advancement in ovarian aging in Indian women.[14] Based on the observation that ART outcomes were less optimal in a population of infertile Indian women, these authors suggested that ethnicity should be considered a risk factor for diminished ovarian reserve and recognized that further research was needed to understand whether these differences are genetically determined or whether other contributing factors such as environment, nutrition, or even lifestyle factors such as sun exposure-related Vitamin D deficiency, play a role.
The aim of this study was to document the ovarian reserve as assessed by measuring serum AMH with an accurate automated method (VIDAS® instrument) and the Vitamin D status, in a large cohort of fertile Indian women covering the reproductive age range (19–50 years).
MATERIALS AND METHODS
Experimental design
We conducted a prospective cohort study of 975 women aged 19–50 years from New Delhi area, who have been invited to participate via a blood donation call from October 2017 to October 2018.
Inclusion and exclusion criteria
To focus on the whole female reproductive lifespan, the criteria for inclusion in this study were fertile Indian women with parity ≥1, age ≥19 years and ≤50 years, and presence of both ovaries. Women diagnosed with PCOS according to the Rotterdam criteria or other endocrine disorders, endometriosis stage III–IV, who underwent adnexal surgery, with tubal ligation or currently using oral contraceptives, were excluded. At enrollment, a questionnaire was filled in recording age, ethnicity, natural parity, and BMI.
This study was approved by an Independent Ethics Committee of the Mother and Child Hospital, and all participants recruited gave their informed consent. Data were gathered anonymously to avoid individual woman identification and kept at Mother and Child Hospital, in New Delhi.
Testing for AMH and Vitamin D
AMH and Vitamin D (25-hydroxyvitamin D) levels were measured in serum. Blood was collected by peripheral venipuncture; the serum was separated from cells by centrifugation, and samples were frozen at −20°C until assayed. All the samples were analyzed in the laboratory at Mother and Child Hospital, as single tests were performed with the automated immunoassays VIDAS® AMH and VIDAS® 25 OH Vitamin D Total assays (bioMérieux, Marcy L’Etoile, France) following the manufacturer’s instructions. For AMH, in case of a concentration above 9 ng/mL, immediate re-testing was performed after 1/4 dilution as recommended. For AMH, the limit of detection (LOD) was determined at 0.01 ng/mL. At concentrations of 0.22, 1.08, 2.99, 5.45, and 7.37 ng/mL, within-lot precision coefficients of variation (CV) were 6.6%, 8.0%, 7.4%, 7.6%, and 8.2%, while within-laboratory precision (between-lot within-instrument) CVs were 8.3%, 9.9%, 9.8%, 8.9%, and 10.6%. In a comparison study with Roche Elecsys® AMH assay, 5.1% CV has been reported for VIDAS® AMH assay.[15] For Vitamin D, the LOD was determined at 8.1 ng/mL. At concentrations of 10.5, 26, and 65.1 ng/mL, within-lot precision CVs were 7.9%, 3.6%, and 1.7%, while within-laboratory precision (between-lot within-instrument) CVs were 16.0%, 4.5%, and 2.8%.
Statistical analysis
For the cohort size, we aimed at targeting 30 women per each year age class, but we finally enrolled 975 subjects, representing age year groups ranging from 16 to 36 individuals. Continuous data are expressed as mean ± standard deviation (SD) and mean. Continuous variables were examined for normality with the Kolmogorov–Smirnov test; if the data were not normally distributed, nonparametric test were used. A normal distribution of values was only observed for age and BMI, so Spearman’s correlation analyses were performed. Statistical analyses were performed with the chi-square test, Fisher exact test, or two-sample Student t test. Linear regression analysis was used to assess the association between variables. To control for confounding factors, stratification was undertaken for age, BMI, AMH, and Vitamin D. Factors that may have a possible impact on the ovarian reserve were assessed with the use of a multivariate analysis that included the potential-related factors: age, BMI, Vitamin D. Significance was set at P < 0.05. Statistical calculations were performed with the use of Statistical Package for the Social Sciences (SPSS) version 21.0. (IBM Corp., Armonk, NY, USA). For establishing age-specific reference intervals for AMH, AMH values have been fitted with a Loess smoothed fit curve (R package) with 95 % confidence interval.
RESULTS
Reference values for the VIDAS® AMH assay have been established for a population of 975 apparently healthy Indian women, aged 19–50 years, with a fertility criteria as defined as at least a natural pregnancy with a living child. This cohort can be considered as representative of the ethnic diversity in the New Delhi region, with a majority of Hindu (80.82%) followed by Muslim (14.67%), Christian (4.0%), and Sikh (0.51%) population. The characteristics (age, BMI, parity) of the women and the AMH and 25(OH) Vitamin D serum concentrations are reported in Table 1. The distribution of ages covers the range from 19 to 50 years, with at least 15 individuals per year age class.
The AMH concentrations observed for these 975 women are shown as a scatterplot [Figure 1]. For 73 of these tested individuals (7.49%), the AMH level was below the LOD for the VIDAS AMH assay (0.01 ng/mL) among whom 74% were 48–50 years old. For 29 individuals (2.97%), AMH first result was above the upper limit of the measuring interval (9 ng/mL), and the exact concentration has been determined with a second measurement after 1/4 dilution. Between the age of 19 and 50 years, the results observed for this cohort show a continuous and somewhat regular decrease of AMH with a global correlation as measured with Spearman’s rank correlation coefficient (rho) of −0.689 (P-value < 0.0001; 95% confidence interval (CI) −0721 to −0655). To better describe the decrease of AMH with age, the values have been fitted with an LOESS smoothed fit curve with 95% CI. None of the results have been removed, and these data can be considered as representative of a fertile women population with additional inclusion criteria solely being healthy status with regular cycles and no past gynecologic history, no endocrine disorders, and no smoking.
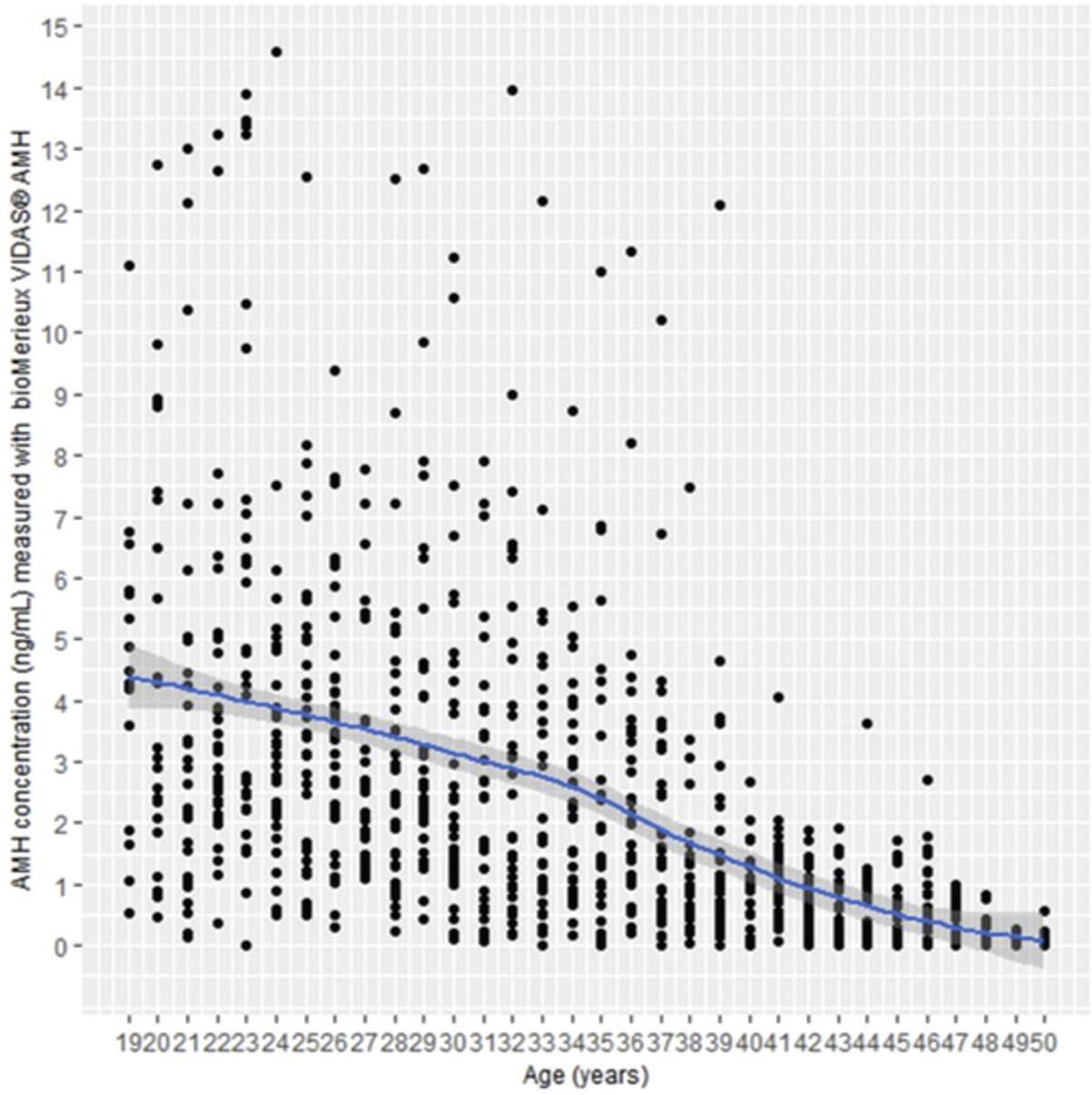
- AMH values observed for the 975 fertile women.
In addition, the AMH values have been analyzed with regards to BMI and Vitamin D status [Table 2]. A negative and statistically significant correlation was observed between AMH and BMI (Spearman’s rho = −0.246 with 95% CI −0.304 to −0.186, and P < 0.0001), but no statistically significant correlation was observed between AMH and Vitamin D (Spearman’s rho = −0.0612 with 95% CI −0124 to 0.00160, P = 0.0561). After univariate linear regression, age and BMI were significant factors affecting AMH, but after adjusting for confounding factors, only age was significantly affecting AMH with P-value < 0.05.

For this population of 975 Indian fertile women, 66.26% of the tested women presented a Vitamin D deficiency—under 20 ng/mL, a cutoff that has been defined by the US Endocrine Society due to consequent and consistent elevation of parathyroid hormone and a decrease in intestinal calcium absorption.[16] A positive and statistically significant correlation was observed between Vitamin D and age (Spearman’s rho = 0.183 with 95% CI 0.122 to 0.243, and P < 0.0001) and between Vitamin D and BMI (Spearman’s rho = 0.163 with 95% CI 0.101 to 0.224, and P < 0.0001). After univariate linear regression, age and BMI were significant factors affecting Vitamin D, but after adjusting for confounding factors, only age was significantly affecting Vitamin D with P-value < 0.05.
DISCUSSION
This study is the first to report AMH concentrations measured with the VIDAS® AMH assay in a large population of fertile Indian women, aged 19–50 years. Indeed, for each age year class, more than 20 women have been tested, representing a reasonable coverage of the inter-individual variability (e.g., personal history, genetic, and environmental factors). Such an approach has allowed to model the regular decrease of AMH from 19 to 50 years. The proposed nomogram, with its 95% CI, is intended to help the clinicians in analyzing any AMH result obtained for an Indian woman of a given age (year by year) to assess her ovarian reserve, for endocrine, gynecology, or fertility purpose.
For good practice, it is important to determine locally the reference values for any new method such as the VIDAS® AMH assay, to help integrating and studying the possible impact of genetics and environmental factors. Several studies have reported such variation for AMH, including one comparing Spanish and Indian women[14] and another one with Mexican populations.[13]This study has also enabled to document the Vitamin D status in 975 Indian fertile women aged 19–50 years, recording a prevalence of 66.26% for Vitamin D deficiency (defined as <20 ng/mL). Previous studies performed in Indians have reported Vitamin D deficiency prevalence mostly ranging from 80% to 90%[17] and in the range of 30% to 91.2% among Indian adults.[18]
Our analysis suggests that after correcting for confounding factors there is no association between BMI and Vitamin D levels. After univariate linear regression, age and BMI were significant factors affecting Vitamin D but after adjusting for confounding factors, only age was significantly affecting Vitamin D with P-value < 0.05. Other studies have shown that levels of Vitamin D are inversely associated with BMI.[17] For this Indian fertile women cohort, the diversity of AMH according to the age was not found to be correlated to the Vitamin D status. Similar results have been observed in a multi-center study in Europe with infertile women.[19]
The AMH values according to age reported in our study are intended to help clinicians, gynecologists, endocrinologists, and fertility specialists to accurately interpret AMH results measured with VIDAS® regarding the inter-individual variability that can be observed in fertile Indian women. The age-related decline of AMH is also reported as a model based on these cross-sectional data analysis across the reproductive age range.
Our study presents several advantages: i) reference values for AMH established with fertile healthy Indian women, ii) the homogeneity of the generated data because of a single center study (same enrollment criteria, same blood sample treatment, single testing with a single VIDAS® system), iii) the quality of the subject enrollment with careful review of health status performed by a single fertility expert gynecologist, and iv) the accuracy of AMH and Vitamin D measurements using the automated VIDAS® assays. This study has however several limitations: i) a cohort size of only 975 Indian women that cannot totally reflect the ethnicity diversity observed in India, even if covering Hindu (80.82%), Muslim (14.67%), Christian (4.0%), and Sikh (0.51%) population and ii) AMH and Vitamin D levels measured were not at the time of natural pregnancy.
Further studies will be required to compare the age-dependent AMH decline between Indian and other populations, and to better understand the contributing factors of ovarian aging.
CONCLUSION
Reference values for AMH have been established for an Indian population to guide the interpretation of results obtained with the automated VIDAS® AMH assay, a simple and robust solution for on-demand testing. The AMH/age nomogram should help clinicians, gynecologists, and fertility specialists to propose individually optimized approaches in case of infertility or other gynecological disorders.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Anti-Müllerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16:113-30.
- [Google Scholar]
- Anti-Müllerian hormone is a promising predictor for the occurrence of menopausal transition. Menopause. 2004;11:601-6.
- [Google Scholar]
- Anti-Müllerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478-83.
- [Google Scholar]
- Anti-Müllerian hormone and inhibin B variability during normal menstrual cycles. Fertil Steril. 2010;94:1482-6.
- [Google Scholar]
- Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update. 2013;19:26-36.
- [Google Scholar]
- Prediction of an excessive response in in vitro fertilization from patient characteristics and ovarian reserve tests and comparison in subgroups: an individual patient data meta-analysis. Fertil Steril. 2013;100:420-9.
- [Google Scholar]
- The meaning of anti-Müllerian hormone levels in patients at a high risk of poor ovarian response. Clin Exp Reprod Med. 2016;43:139-45.
- [Google Scholar]
- The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenauposal women with breast cancer. Hum Reprod. 2006;21:2583-92.
- [Google Scholar]
- Pretreatment serum anti-Müllerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96:1336-43.
- [Google Scholar]
- Pretreatment anti-Müllerian hormone levels determine rate of posttherapy ovarian reserve recovery: acute changes in ovarian reserve during and after chemotherapy. Fertil Steril. 2013;99:477-83.
- [Google Scholar]
- Anti-müllerian hormone in the pathophysiology and diagnosis of polycystic ovarian syndrome. Curr Opin Endocrinol Diabetes Obes. 2018;25:377-84.
- [Google Scholar]
- Variation in levels of AMH among Maya and non-Maya women in Campeche, Mexico. Am J Phys Anthropol 2018:1-9.
- [Google Scholar]
- Ethnicity as a determinant of ovarian reserve: differences in ovarian aging between Spanish and Indian women. Fertility and Sterility. 2014;102:244-9.
- [Google Scholar]
- New AMH assay allows rapid point of care measurements of ovarian reserve. Gynecol Endocrinol. 2017;33:638-43.
- [Google Scholar]
- Evaluation, treatment, and prevention of Vitamin D Deficiency: an endocrine society clinical practice guideline. J Clin Endocrin Metab. 2011;96:1911-30.
- [Google Scholar]
- Vitamin D deficiency in India. Journal of Family Medicine and Primary Care. 2018;7:324-30.
- [Google Scholar]
- Prevalence of hypovitaminosis D in India & way forward. Indian J Med Res. 2018;148:548-56.
- [Google Scholar]
- The effect of serum Vitamin D levels on ovarian reserve markers: a prospective cross-sectional study. Hum Reprod. 2017;32:208-14.
- [Google Scholar]







