Translate this page into:
Effect of autologous platelet-rich plasma on clinical outcome in patients with suboptimal endometrium in fresh ICSI/FET cycle − an interventional prospective study
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Endometrial receptivity (ER) is crucial for implantation rate and clinical pregnancy rate in ET-IVF. Endometrial receptivity is affected independently by endometrial thickness, vascularity and pattern.
Aims:
To evaluate the efficacy of autologous platelet-rich plasma in improving the endometrial parameters in patients with suboptimal endometrium in fresh ICSI/FET cycle. To evaluate pregnancy outcome in FET/ICSI cycles after intrauterine instillation of PRP.
Settings and Design:
Interventional prospective study conducted in tertiary infertility centre.
Methods and Material:
30 patients with suboptimal endometrium undergoing IVF-ICSI/FET cycle with failed implantation/history of previous cycle cancellations/intrauterine adhesions on hysteroscopy were included. Patients with poor embryo quality, Bleeding dyscracias, Low platelet counts or active infections were excluded. All patients with any compromised endometrial parameter were offered intra-uterine PRP infusion.
Statistical analysis used:
Categorical data were presented as frequency and percentage; for comparison Chi square and McNemar tests were used wherever applicable for calculation of P-value. P-value < 0.05 was considered significant.
Results:
Endometrial thickness showed good response to PRP therapy and the mean increase in endometrial thickness was 1.33mm. PRP therapy significantly changed the endometrial pattern from Type A to Type B in more than half the patients (P-value = 0.001). PRP was instrumental in improving the sub-endometrial flow (22/30 patients, 73.3%) and uterine artery resistance (16/20, 80%), more so in subgroup of patients suffering from Intrauterine adhesions (P-value = 0.004 and 0.001 respectively). 43.3% (13/30) showed positive β-hCG. A clinical pregnancy rate of 30% (9/30) was seen.
Conclusions:
PRP has promising results in patients with suboptimal endometrium by increasing endometrial thickness, ameliorating pattern and improving vascularity with reduced cancellation rate.
Keywords
Clinical pregnancy rate
endometrial pattern
endometrial thickness
endometrial vascularity
PRP
sub-optimal endometrium
uterine artery resistance
Key Messages: In patients with intrauterine adhesions and repeated implantation failure due to poor endometrial parameters, Intrauterine instillation of PRP can be tried before suggesting the option of surrogacy.
INTRODUCTION
Endometrial receptivity (ER) is crucial for implantation rate and clinical pregnancy rate in In-vitro fertilization (IVF). Endometrial receptivity is affected independently by endometrial, vascularity and pattern.[1] Suboptimal endometrium has been associated with poor clinical outcome[2,3,4,5] and unexpected cryopreservation of embryos in already anxious subfertile patients.[1] Defining the parameters for optimal endometrium for embryo transfer is a challenge faced by Assisted Reproductive Technology (ART) practitioners.
Studies have also stated that lack of identifiable blood flow in the endometrial and sub-endometrial regions a marker of poor implantation. The growth of the endometrium lining is dependent upon the quality of blood flow to the uterus as well as the effect of estrogen in encouraging the lining to develop. In patients with sub-optimal endometrial lining, conventionally many therapies have been tried, such as stronger dose or protracted use of estrogen, Sildenafil citrate, superadded low dose aspirin, intrauterine instillation of G-CSF (Granulocyte colony-stimulating factor), beta-Human Chorionic Gonadotropin (βhCG), as well as certain nonconventional therapies such as electro-acupuncture and scratching, but they lack consistency in delivering results.[6,7,8,9,10,11,12,13,14] There is a need to evaluate other modalities in this regard, as suboptimal endometrial growth or vascularity is known to cause repeated cycle cancellations and recurrent implantation failure, thus causing not just psychological but also financial impact on patients. This drives the patients toward surrogacy as an option, which, considering the medicolegal implications, might not be a viable option now.[1]
Additionally, patients with intrauterine adhesions have shown poor response to standard treatment for thin endometrium.[15,16] Intrauterine instillation of PRP is a novel approach for the management of refractory endometrium.[15] However its efficacy is debated because of lack of randomised control trials. There is paucity of literature so the aim of the present study is to evaluate the effectiveness of intrauterine instillation of PRP in the management of suboptimal endometrium in Frozen embryo transfer (FET)/fresh Intracytoplasmic sperm injection(ICSI) cycles.
SUBJECTS AND METHODS
The study was conducted over a period of 6 months from August 2018 to January 2019 at a tertiary IVF centre after approval by the Independent Ethics Committee and after obtaining written informed consent from patient. Subjects were enrolled taking into consideration the inclusion and exclusion criteria.
Inclusion criteria
Subjects undergoing IVF-ICSI//FET were included if they fulfilled the following criteria.
Subjects with failed implantation with suboptimal endometrial parameters in previous cycle.
Subjects with history of cycle cancellations due to sub-optimal endometrium in previous cycle.
Suboptimal endometrial parameters in patients in fresh ICSI or FET cycle.
Subjects with intrauterine adhesions documented by hysteroscopy.
Exclusion criteria
Patients with poor embryo quality
Bleeding dyscracias
Platelet counts < 105/μL
Haemoglobin concentration< 10 g/dl
Other concomitant active infection
In the study population, hysteroscopic assessment of uterine cavity was done in all patients, if not done previously. If any pathology was diagnosed, correction was done. If an area of hyperaemia, focal atrophy, pale endometrium or stromal edema or micro-polyps were found as per Cicinelli (2005) criteria,[17] diagnosis of endometritis was made and confirmed by histopathological analysis.
In subjects with intra-uterine adhesions (IUA), they were classified into minimal, moderate or severe categories based on uterine cavity involvement as per March et al (1978).[18] More than ¾th uterine cavity involvement was defined as severe grade (Asherman’s syndrome). It was considered normal if negative for endometrial pathology.
ICOS (Individualised Controlled ovarian stimulation) was done in all patients planned for fresh ICSI cycle. For FET cycle endometrial preparation was done using Hormone replacement therapy (HRT) for all patients using estradiol valerate (Emgra; Emcure Pharmaceuticals Ltd, India) 6 mg/day in three divided doses, started on the day 2/day 3 of the menstrual cycle. This was followed by serial transvaginal ultrasound examinations. Dose of estradiol was increased as indicated by serial ultrasound examinations upto 12mg/day in divided doses.
Subjects planned for fresh ICSI cycle in whom individualised controlled ovarian stimulation was done, were called for regular TVS to assess the size of follicles and endometrial thickness on day 8 of menstrual cycle then further monitoring according to the patient response done by same ultrasonologist (who was blinded for use of PRP) using 7–9 MHZ multifrequency probe to prevent inter-observer variation.
Similarly patients for FET were called for endometrial evaluation.
Endometrial parameters (thickness, morphology and vascularity) were assessed. ET was measured by transvaginal ultrasound in the plane through the mid-sagittal longitudinal axis of the uterine body as the maximum distance between two echogenic interfaces of the myometrium and endometrium. Average of three measurements was taken and recorded.
Morphology was graded as follows:
Grade A − multilayered with intervening area more echogenic than myometrium.
Grade B − multilayered with intervening area hypoechoic to myometrium.
Grade C − homogenous hyperechoic endometrium
The vascularity of endometrium was classified using Applebaum criteria.[19]
Uterine artery flow was assessed; RI-0.60-0.80 and PI-2.22-3.16 were considered as normal values of uterine indices.[19]
Subjects with any compromised endometrial parameters (endometrial thickness <7mm, high resistance flow in uterine vessels or RI > 3.2, poor vascularity in zone 3and 4 or regular pattern of endometrium) were offered intra-uterine PRP infusion.
Preparation of PRP: PRP was prepared from autologous peripheral blood by centrifugation in two steps as illustrated in Fig. 1. This procedure made 7–10 times concentration of platelets in the final preparation compared to blood which gave appropriate quantities of growth factors. PRP volume of 0.5 to 0.8 ml was thus obtained and infused into the uterus using an intra-uterine insemination (IUI) cannula, introduced gently without touching the endometrium and was administered by slow intrauterine infusion following all aseptic precautions.

- Preparation of autologous PRP from peripheral venous blood in a two-step centrifuge process.
In fresh ICSI, PRP infusion was performed on day of trigger in all the subjects with suboptimal endometrium and it was repeated after ovum pick up.
During FET cycle, PRP infusion was done 2 days before progesterone and repeated on the day of progesterone if required. When the endometrial thickness crossed 7 mm with good vascularity pattern, injection Uterone100 mg/ Aquasusten 25mg once −a day was started and embryo transfer (ET) was carried out per embryonic stage. On the day of embryo transfer endometrial parameters (subendometrial flow, uterine artery resistance, thickness and pattern) were evaluated. If endometrial thickness was found to be less than 6 mm after all adjuvants, no embryo transfer was carried out.
After the ICSI/FET procedures luteal phase support was given. After 15 days subjects were called for β-hCG estimation for the confirmation of the pregnancy.
Clinical pregnancy was diagnosed two weeks after the β-hCG estimation by the presence of the fetal cardiac activity.
β-hCG more than 50 mIU was diagnosed as positive
RESULTS
In the study group, 40% of subjects were below 30 years while rest were over 30 years of age. 33.3% subjects were married for ≤ 3 years while remaining had a married life of more than 3 years.
Among the study subjects, majority of the patients 56.7% (17/30) had normal menstrual cycles. 30%(9/30) and 13.3%(4/30) subjects had oligomenorrhoea and secondary amenorrhoea respectively. More than half 56.7% (17/30) of the subjects in the study population had a previous history of tuberculosis. All of them had received treatment for the same and were declared cured.
More than half of the study subjects presented for treatment of primary infertility (16/30). Intrauterine adhesions syndrome and tubal factors were the most common cause of infertility among study subjects occurring in more than half of them.
Among the 27/30 subjects who had atleast one prior failed IVF/ICSI cycle, 20 subjects had received G-CSF, while four had received ecosprin and Sildenafil combination and three subjects were treated with ecosprin alone.
Among the 27/30 subjects who had a previous failed IVF/ICSI cycle, 12/27 subjects had a history of cycle cancellation due to suboptimal endometrium.
Majority of the subjects had intrauterine adhesions (18/30) as the cause of suboptimal endometrium, of which 10 had Asherman syndrome. 26.7%(8/30) subjects displayed endometritis on hysteroscopy and 13.3% (4/30) subjects had normal hysteroscopy findings.
In the present study, equal number of subjects underwent FET & ICSI cycles(15 subjects each).
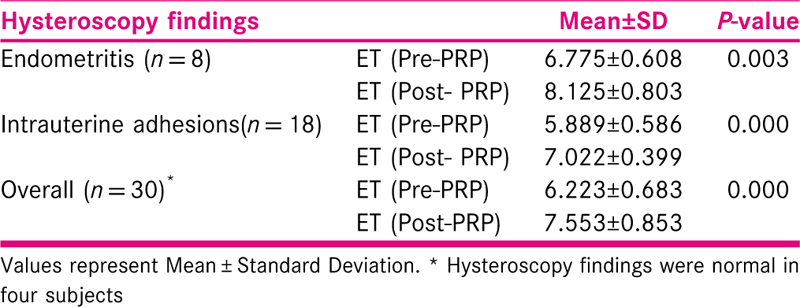
The endometrial thickness in subjects before and after intra-uterine instillation of PRP was recorded and it was found that there was statistically significant increase in the endometrial thickness after PRP therapy. The mean endometrial thickness before and after PRP therapy was found to be 6.223 mm and 7.553 mm respectively. This significant difference was seen in both − endometritis and intrauterine adhesions subgroup of subjects, mean thickness after PRP was 8.125 mm in subjects with endometritis and 7.022 mm in subjects with intrauterine adhesions [Table 1].
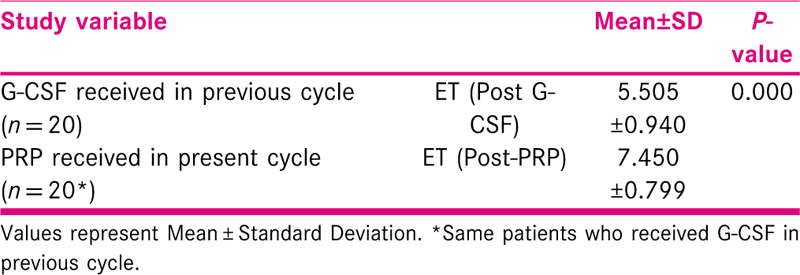
In the subgroup of subjects who had received G-CSF (n = 20) in the previous cycle for sub-optimal endometrium, there was statistically significant difference in endometrial thickness after PRP therapy. Overall, in 27/30 subjects who had a previous failed IVF/ICSI cycle,12/27 subjects had a history of cycle cancellation due to suboptimal endometrium, there was significant increase in the endometrial thickness after PRP therapy [Table 2].
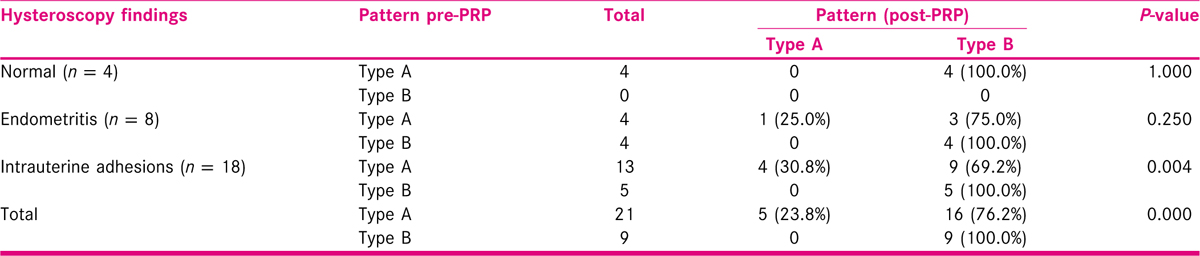
The endometrial pattern was observed in all subjects before and after PRP therapy. 53.3% patients were benefitted by PRP therapy in which endometrial pattern changed from Type A to Type B. 16.7% subjects were refractory to PRP. This difference was found to be statistically significant. The significant difference in change of endometrial pattern was seen in the subgroup of patients suffering from intrauterine adhesions syndrome. Those patients who had normal hysteroscopy findings or had endometritis did not show statistically significant improvement in the endometrial pattern [Tables 3 and 4].

In 22/30 subjects (73.3%) there was increase in the sub-endometrial blood flow poor to good grade and no change in grade was observed in 4/26 subjects with poor sub-endometrial flow. Overall, there was statistically significant increase in the sub-endometrial flow in post-PRP ultrasound [Table 5].

The significant difference in change of endometrial flow was seen in the subgroup of subjects suffering from intrauterine adhesions syndrome. Those subjects who had normal hysteroscopy findings or had endometritis did not show statistically significant improvement in the endometrial blood flow [Table 6].

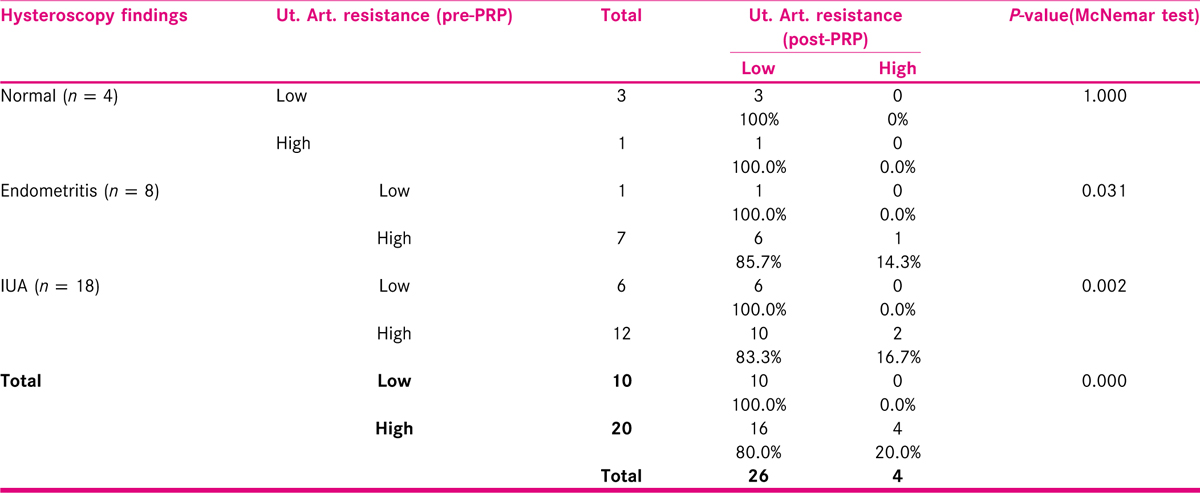
PRP therapy significantly reduced uterine artery resistance in 16/20 (80%) of patients in this study. In subgroup analysis, it was found that this difference was seen in subgroup of patients (10/12, 83.3%) with intrauterine adhesions [Table 7].
43.3% (13/30) showed positive β-hCG rates after PRP therapy. 61.1% (11/18) subjects with intrauterine adhesions showed positive beta-hCG results. A clinical pregnancy rate of 30% (9/30) was seen in this study. 7/18 subjects with intrauterine adhesions showed positive clinical pregnancy. Out of nine clinical pregnancies, six ongoing pregnancies are in their second trimester. One of the subjects had a missed abortion at 9 weeks. Two subjects are progressing normally in their first trimester [Figure 2].

- Comparison of positive β-hCG and clinical pregnancy rate in the study.
DISCUSSION
Our study aimed at analysing the effect of intra-uterine instillation of PRP in subjects with sub-optimal endometrial parameters undergoing IVF/ICSI or FET cycles. The effect of PRP therapy was observed on endometrial thickness, endometrial pattern, subendometrial vascularity and uterine artery Doppler parameters. Biochemical and clinical pregnancy rates were also recorded.
PRP and ET: In our study the mean difference in endometrial thickness before and after PRP therapy was found to be 1.33. This significant difference was seen in both − Endometritis and IUA subgroups. Mean ET in our study was 7.55 mm which was comparable to 7.22 mm, 6.9 mm and 7 mm in a study by Tandulwadkar[16], Colombo[20] and Chang[15] respectively.
PRP vs G-CSF: 20 patients who had received G-CSF in the previous unsuccessful cycle, acted as their controls and showed statistically significant increase in ET from the previous cycle. The present study is the first study to compare the effect of G-CSF & PRP on the endometrium.
PRP and Endometrial pattern: 16/21 (76.1%) patients were benefitted by PRP therapy in which endometrial pattern changed from Type A to Type B. Colombo et al.[20] also reported an improvement in endometrial pattern after PRP in 7/8 (83.5 %) patients.[20] This difference can be attributed to larger study population (30 vs 8) and inclusion of patients with IUA in our study, in contrast to Colombo et al.[20] who had excluded these patients.
PRP and Sub-endometrial blood flow: In 26/30 subjects (86.7%), there was increase in the sub-endometrial blood flow − poor to good grade. This was comparable to results of the study by Tandulwadkar[16] who reported increase in 94.1% patients. The significant difference in change of endometrial pattern was seen in the subgroup of subjects with intrauterine adhesions. PRP and Uterine artery resistance: 16/20 (80 %) patients had lower uterine artery resistance after PRP therapy. In subgroup analysis, it was found that this difference was seen in subjects with intrauterine adhesions. To the best of our knowledge, no previous study had assessed the effect of PRP on uterine artery resistance.
PRP and regnancy
The positive beta-hCG and clinical pregnancy were lower than a previous study by Tandulwadkar (2017), 43.3% vs 60.93% and 30% vs 45.31% respectively.[16] Chang (2015) reported a clinical pregnancy rate of 80%.[15] Higher difference in pregnancy was probably due to difference in selection criteria which excluded poor prognosis patients and less number of patients in these studies.
11/18 patients had positive β-hCG, but clinical pregnancy was observed in only seven patients. It was thus observed that PRP therapy increased positive β-hCG in patients with IUA, however the clinical pregnancy outcome is not significantly affected in these patients.
Strength of the study
The strength of our study is objective analysis of two additional vital characteristics of endometrium, the sub-endometrial flow and uterine artery resistance along with endometrial thickness.
We included a significant number of patients with poor prognosis due to subendometrial factors, importantly severe IUA, in whom all previous therapies (surgical correction, Sildenafil, G-CSF) were unsuccessful. All previous studies have excluded this important poor prognostic group, which is rampant in our population and thus clinically significant.
Comparison between PRP and G-CSF (received in previous cycle) was done in our study, with patients acting as their own controls.
Limitations
Due to short study duration, lesser number of patients could be included in the study. Also, for the same reason, clinical pregnancies could not be followed for long term.
Summary
We conclude that PRP has promising results in patients with suboptimal endometrium by increasing endometrial thickness, ameliorating pattern and improving vascularity. It reduces the cancellation rate of ET-IVF due to poor endometrium and avoids third-party surrogacy in some cases. Preparation of PRP is a rather simple procedure and a financially viable option. PRP is autologous and thus an immunologically safe option. In our study, PRP performed better than G-CSF in suboptimal endometrium. PRP performed better in patients with Intra-uterine adhesions than in patients with endometritis or with normal hysteroscopy findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The endometrium in assisted reproductive technology: how thin is thin? J Hum Reprod Sci. 2016;9:3-8.
- [Google Scholar]
- Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:530-41.
- [Google Scholar]
- Endometrial pattern, but not endometrial thickness, affects implantation rates in euploid embryo transfers. Fertil Steril. 2015;104:620-8.
- [Google Scholar]
- Endometrial vascularity: its relation to implantation rates. Int J Infertil Fetal Med. 2012;3:48-50.
- [Google Scholar]
- Correlation of subendometrial-endometrial blood flow assessment by two-dimensional power Doppler with pregnancy outcome in frozen-thawed embryo transfer cycles. J Hum Reprod Sci. 2014;7:130-5.
- [Google Scholar]
- Extended estrogen administration for women with thin endometrium in frozen-thawed in-vitro fertilization programs. J Assist Reprod Genet. 2006;23:337-42.
- [Google Scholar]
- Aspirin in women undergoing in vitrofertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2007;88:822-31.
- [Google Scholar]
- Endometrial growth and uterine blood flow: a pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertil Steril. 2010;93:1851-8.
- [Google Scholar]
- Effect of vaginal Sildenafil on the outcome of in vitro fertilization (IVF) after multiple IVF failures attributed to poor endometrial development. Fertil Steril. 2002;78:1073-6.
- [Google Scholar]
- Evaluation of granulocyte colony-stimulating factor effects on treatment-resistant thin endometrium in women undergoing in vitro fertilization. Biomed Res Int. 2014;2014:913235.
- [Google Scholar]
- A pilot cohort study of granulocyte colony-stimulating factor in the treatment of unresponsive thin endometrium resistant to standard therapies. Hum Reprod. 2013;28:172-7.
- [Google Scholar]
- A randomized clinical trial of endometrial perfusion with granulocyte colony stimulating factor in in vitro fertilization cycles: impact on endometrial thickness and clinical pregnancy rates. Fertil Steril. 2014;101:710-5.
- [Google Scholar]
- Electroacupuncture reduces uterine artery blood flow impedance in infertile women. Taiwan J Obstet Gynecol. 2009;48:148-51.
- [Google Scholar]
- Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 2015;8:1286-90.
- [Google Scholar]
- Autologous intrauterine platelet-rich plasma instillation for suboptimal endometrium in frozen embryo transfer cycles: a pilot study. Journal of Human Reproductive Sciences. 2017;10:208-12. doi:10.4103/jhrs.JHRS_28_17
- [Google Scholar]
- Endometrial micropolyps at fluid hysteroscopy suggest the existence of chronic endometritis. Hum Reprod. 2005;20:1386-9.
- [Google Scholar]
- Hysteroscopic management of intrauterine adhesions. Am J Obstet Gynecol. 1978;130:653-7.
- [Google Scholar]
- The steel and Teflon endometrium − ultrasound visualization of the endometrial vascularity in IVF patients and outcome. Presented at the third world congress of ultrasound in Obstetrics and Gynecology. Ultrasound Obstet Gynecol. 1995;6:191-8.
- [Google Scholar]
- Use of platelet rich plasma in human infertility. J Biol Regul Homeost Agents. 2017;31:179-82.
- [Google Scholar]







