Translate this page into:
Effect of hyaluranon-rich medium for embryo transfer on IVF outcome in patients with recurrent implantation failure
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
The aim of the study was to evaluate efficacy of hyaluronan-rich medium on implantation and clinical pregnancy rates in vitrified-warmed blastocyst transfers in recurrent implantation failure patients.
Methods:
This was a randomized controlled study conducted at Akanksha IVF Centre, New Delhi. One hundred patients with history of previous two or more implantation failures, with at least one implantation failure at the present center, were included in the study. Patients were randomly divided into two groups: study group (n = 37) had embryos transferred into 20 μL of EmbryoGlue for 10 minutes prior to transfer inside uterine cavity. In the control group (n = 31), embryos were transferred to the conventional blastocyst culture medium. Statistical analysis was performed using Statistics Package for Social Sciences.
Results:
The patient’s mean age, causes of infertility, serum anti-Müllerian hormone level, embryo quality and the number of transferred embryos were comparable between the study and control groups. The clinical pregnancy rate in study group was higher than control group (40.5% vs 25.8%). However, the difference was not statistically significant (P = 0.58). There was no statistically significant improvement in implantation rate (44% vs 34.1%; P = 0.32), miscarriage rate (6.6% vs 12.5%; P = 0.27) and multiple pregnancy rate (13.3% vs 12.5%; P = 0.9).
Conclusion:
The use of EmbryoGlue in transfer medium in the blastocyst transfer in vitrified-warmed cycles does not have any significant effect on the implantation and pregnancy rates in patients with previous implantation failure.
Keywords
Frozen-thawed embryo transfer
hyaluronan-rich transfer medium
recurrent implantation failure
INTRODUCTION
Nowadays assisted reproductive technologies have become an integral part of infertility treatment. To increase the efficacy of in vitro fertilization/embryo transfer (IVF‑ET) cycles, advances have been made in ovarian stimulation protocols, technique of fertilization, embryo culture, and transfer mediums along with many other aspects of this treatment. Improvement in human embryo culture technology in the form of better culture media and culture systems has led to an increase in blastocyst formation, clinical pregnancy, rate and live birth rate.[1] Despite all these improvements, implantation of embryos remains a challenge to the clinician. Several factors that contribute to embryo development and implantation have not yet been determined. Implantation is a complex process that includes zona hatching, apposition, and adhesion of blastocyst followed by an invasion of trophoblast into the uterine endometrium.[2] One of the various reasons for unsuccessful implantation is failure to develop a sticky matrix between blastocyst and endometrium. Much of the research has been done on the interaction between the embryo and the endometrium at the time of implantation. As the composition of the medium that surrounds the embryo at the time of transfer is considered to be crucial, various studies have focused on the effect of adding a specific adherence compound like hyaluronan (HA) to the ET medium on the implantation rate.[3,4] HA, a glycosaminoglycan, is present in the oviduct and uterine fluid and increases at the time of implantation. EmbryoGlue is a human ET medium, which contains high concentration of HA (0.5 mg/mL) and low concentration of recombinant human albumin (rHA = 2.5 mg/mL). Gardner et al.[3] observed that HA supplementation results in the highest implantation rate and fetal development after blastocyst transfer in mice. Cochrane review in 2010 also found higher clinical pregnancy rate in the group treated with hyaluronic acid.[4] However, Fancsovits et al.[5] did not find any significant difference in clinical pregnancy rate (42.4 vs 39.2%), implantation rate (23.3 vs 23.2%), and delivery rate (31.0 vs 29.2%) between the HA group and the control group. In another study, Singh et al.[6] found a significantly higher implantation rate (50% vs 0%, P = 0.04) in patients with previous IVF failure. Hence, the study was done to evaluate the efficacy of HA-rich transfer medium in vitrified-warmed blastocyst transfer on the implantation and pregnancy rate in patients with a history of previous implantation failure.
MATERIAL AND METHODS
A prospective, randomized controlled study including 68 patients was conducted at Akanksha IVF Centre from January 2019 to September 2019. All patients, between 26 and 37 years of age, with history of previous two or more implantation failures and with at least one episode of previous ET failure at Akanksha IVF Centre were included in the study. These patients were planned for vitrified-warmed blastocyst transfer within a hormone replacement cycle.
Exclusion criteria:
Patients with age >37 years
Uterine fibroid/adenomyosis
Donor cycles
Abnormal semen parameters [oligozoospermia, severe oligoasthenoteratozoospermia (OATS)]
The randomization to the study and control groups was performed using a computerized randomization table. In study group (n = 37), the ET included HA-rich medium (EmbryoGlue), and in control group (n = 31) a conventional transfer medium was used.
Ovarian stimulation protocol
The GnRH antagonist fixed protocol (Cetrotide; Serono, London, UK) was used for ovarian stimulation. Stimulation with 225 International Units (IU) of recombinant follicle stimulating hormone (FSH) (Gonal F; Serono, Switzerland) was started and from the sixth day of stimulation, cetrorelix were injected subcutaneously. Once the majority of follicles (at least 3) reached ≥ 18 mm, serum oestradiol was measured and ovulation trigger was administered (Inj. decapeptyl, 100 μl/mL; Silverline Medicare Pvt. Ltd, Maharashtra). Ovum pickup was performed after 34-36 hours of ovulation trigger. Ova-Stiff Echo Tip Single Lumen Ovum Aspiration Needle (Cook’s medical, Australia) was used for ovum pickup.
In vitro fertilization procedure
Fertilization of oocytes was performed using classical IVF or intracytoplasmic sperm injection. Embryos were cultured to blastocyst stage in single step media (SAGE 1 step; CooperSurgical Fertility, Denmark) and daily quality assessment was done. Vitrification of blastocysts was performed according to standard protocol.[7]
Endometrial preparation
The uterine endometrium was prepared using oral estradiol valerate (Tab. Progynova 2 mg, Bayer PLC, United Kingdom) twice daily, administered from the second day of the menstrual cycle, and increasing the dose according to endometrial thickness as assessed on day 7, 11, and 14 of the cycle. Progesterone (100 mg in oil; Inj uterone, Jagsonpal Pharmaceuticals, New Delhi, India) was initiated on the 14th day when the endometrial thickness was ≥7 mm and both the hormones were continued till the day of the urinary pregnancy test.
Procedure of embryo transfer
After warming, good quality blastocysts were selected on the morning of day 5 by blastocyst grading criteria described by Gardner and Schoolcraft.[8] HA-rich transfer medium (EmbryoGlue, Vitrolife, Sweden; 1.5 mL) was incubated at 37°C in 6% CO2 for 12-24 hours before ET. From this incubated media, 1 mL was used to make transfer dish and 0.5 mL to load the syringe for catheter. Embryos in the study group were placed in this transfer dish for 20 minutes before the transfer. In control group, the conventional transfer medium (1.5 mL, SAGE single step media) was incubated at 37°C in 6% CO2 for 12-24 hours before ET and embryos were placed in this transfer dish for 5 to 10 minutes before the transfer. ET catheter (Sydney IVF embryo transfer set, cook’s medical, USA) loaded with embryos by sandwich method, making a total volume of 20 μL, was used to place embryos in uterine cavity approximately 1.5 cm below the fundus, as seen on transabdominal ultrasound. Luteal support was given with oral oestradiol valerate and injectable progesterone. Pregnancy was confirmed by a serum β-human chorionic gonadotropin test, 14 days after blastocyst transfer.
Outcome measures
The primary outcome was the implantation rate defined as the number of gestational sacs confirmed by transvaginal ultrasonography divided by the number of embryos transferred and the clinical pregnancy rate defined as the number of patients with at least one gestational sac divided by the number of transfers. Secondary outcome included miscarriage rate defined as the number of clinical pregnancy losses, including ectopic pregnancy before the 20th week of gestation, divided by the total number of clinical pregnancies.
Ethical justification
This study was conducted on ethical guidelines for biomedical research on human subject as given in the “Declaration of Helsinki” and by Central Ethics Committee on Human Research of ICMR, New Delhi. It has been approved by the institutional ethical committee of Mata Chanan Devi Hospital, Janakpuri, New Delhi. A written and informed consent was taken from all the participants and were given the right to opt out at any time during the course of the study without any impact on the treatment to be given.
Data analysis
Statistical analysis was performed using Statistics Package for Social Sciences (SPSS) for Windows version 17 (SPSS, Inc., Chicago, USA). Student t test was used to compare normally distributed parametrical variables and Mann–Whitney test for nonnormally distributed variables. The chi-square or Fisher exact test was used to compare categorical variables. Statistical significance was set at P < 0.05.
RESULTS AND OBSERVATIONS
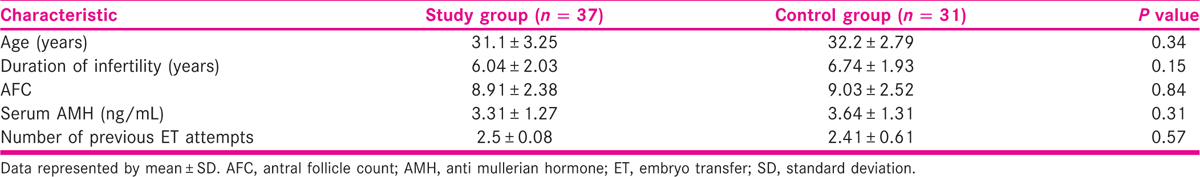
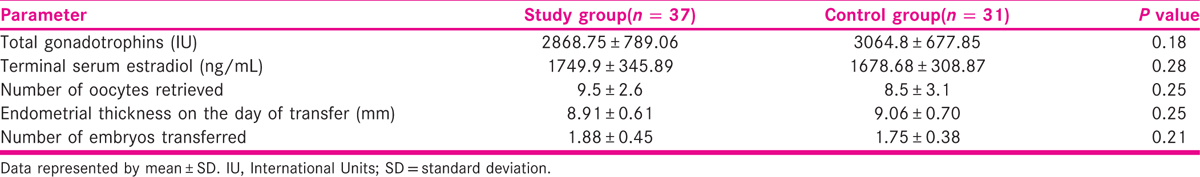
Thirty-seven patients in the study group and 31 patients in the control group completed the study protocol [Figure 1]. Baseline patient characteristics for the study and control groups are summarized in Table 1. The average age, duration of infertility, serum anti mullerian hormone (AMH), and number of previous ET failures were similar among study and control group patients. There were no significant differences in the total dose of gonadotropins, number of oocytes collected, number of transferred embryos, and endometrial thickness between the two groups as depicted in Table 2.

- Consort flow diagram of participants through each stage of randomized trial
Primary and secondary outcome
The implantation rate of blastocyst in the study and control group was 44% and 34.1%, respectively. Similarly, the clinical pregnancy rate was higher in the study group compared to the control group (40.5% vs 25.8%). However, the difference was not statistically significant [Figure 2]. The miscarriage rate was lower in the study group (6.6% vs 12.5%, P = 0.27) than in control group, although it was statistically insignificant. There was no statistical difference in the multiple pregnancy rate (13.3% vs 12.5%, P = 0.9).
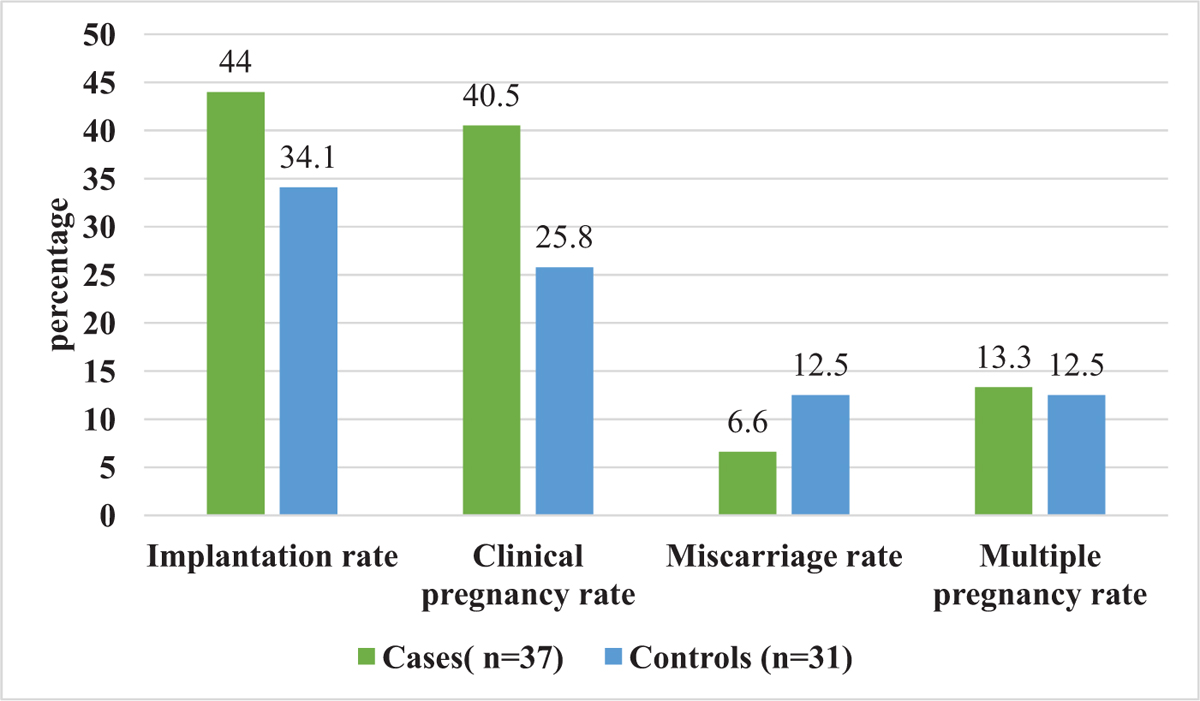
- Comparison of clinical outcomes between the study and control groups
DISCUSSION
HA is naturally present in the female reproductive organs, mainly in the fallopian tubes and uterine cavity as well as in cervical mucus, cumulus, and follicular fluid. It forms a viscous solution that enhances the ET process and prohibits embryo expulsion.[3] Also, the synthesis of HA is induced at the time of implantation and its action is mediated via CD44 receptors. This process appears to be important for embryonic development.[9] In a retrospective study including all the patients undergoing IVF, Safari et al.[10] did not find any significant benefit of HA-rich transfer medium when used in all patients. Therefore, we included patients with previous two or three implantation failures. After analysis of the 16 studies in 2014, Bontekoe S et al.[4] found that by using higher concentrations of HA, the chances of pregnancy and live birth (450 vs 367 per 1000) increased (moderate quality evidence). In the present study, patients in the EmbryoGlue group had higher implantation, clinical pregnancy, and lesser abortion rate as compared to the patients in control group. However, the difference was not statistically significant. Similar results were found in other studies.[5,11,12,13] A study by Ruane et al.[14] found that when HA-treated (for 10 minutes) artificially hatched day 6 blastocysts were cocultured with endometrial cells, there was no significant difference in early or late attachment as compared to controls. This further supports our finding. Even in donor frozen-thawed ET cycles, done after preimplantation genetic testing, EmbryoGlue does not improve IVF outcome as observed in a study.[15] On the contrary, in a study comparing clinical pregnancy and implantation rate among patients receiving either fresh ET or frozen thawed (natural and replacement cycle), the pregnancy rate (37.5%, 31.4%, and 41.2%, respectively) was significantly higher when using HA enriched transfer medium as compared to control medium (10.9%, 10.0%, and 15.7%, respectively; P < 0.05).[16] Korosec et al.[17] found the use of HA-rich medium to improve pregnancy rate in fresh blastocyst transfer and not in frozen cycles. Similarly, a study, which included only day 3 frozen ET cycles accompanied by assisted hatching in all cases, observed improvement in implantation and clinical pregnancy rate with the use of HA-rich transfer medium.[18]
CONCLUSION
The use of HA-rich transfer medium for blastocyst transfer in vitrified-warmed cycles does not improve the implantation and pregnancy rates in patients with previous implantation failure significantly. Similar studies with larger sample size should be done for robust conclusions.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Textbook of Assisted Reproductive Techniques. In: Gardner DK, Weissman A, Howles CM, Shoham Z, eds. Clinical Perspectives Vol Volume 2. (5th). USA: CRC Press; 2018. p. :662-68.
- [Google Scholar]
- Recurrent implantation failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. 2018;16:121.
- [Google Scholar]
- Fetal development after transfer is increased by replacing protein with the glycosaminoglycan hyaluronan for mouse embryo culture and transfer. Hum Reprod. 1999;14:2575-80.
- [Google Scholar]
- Adherence compounds in embryo transfer media for assisted reproductive technologies. Cochrane Database Syst Rev. 2014;2014:CD007421.
- [Google Scholar]
- Effect of hyaluronan-enriched embryo transfer medium on IVF outcome: a prospective randomized clinical trial. Arch Gynecol Obstet. 2015;291:1173-9.
- [Google Scholar]
- Role of Embryo Glue as a transfer medium in the outcome of fresh non-donor in-vitro fertilization cycles. J Hum Reprod Sci. 2015;8:214-7.
- [Google Scholar]
- Comparison of clinical outcomes between single and double vitrified-warmed blastocyst embryo transfer according to the day of vitrification. J Assist Reprod Genet. 2013;30:779-85.
- [Google Scholar]
- Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307-11.
- [Google Scholar]
- Hyaluronan regulates cell behaviour: a potential niche matrix for stem cells. Biochem Res Int. 2012;2012:1-11.
- [Google Scholar]
- Routine use of EmbryoGlue(®) as embryo transfer medium does not improve the ART outcomes. Arch Gynecol Obstet. 2015;291:433-7.
- [Google Scholar]
- Hyaluronan-enriched transfer medium in cleavage-stage frozen-thawed embryo transfers increases implantation rate without improvement of delivery rate. Fertil Steril. 2010;94:1669-73.
- [Google Scholar]
- Efficacy of hyaluronan-rich transfer medium on implantation and pregnancy rates in fresh and frozen-thawed blastocyst transfers in Korean women with previous implantation failure. Obstet Gynecol Sci. 2016;59:201-7.
- [Google Scholar]
- Evaluation of transfer media containing different concentrations of hyaluronan for human in vitro fertilization. Reprod Med Biol. 2017;16:349-53.
- [Google Scholar]
- The effects of hyaluronate-containing medium on human embryo attachment to endometrial epithelial cells in vitro. Hum Reprod Open. 2020;2020:hoz033.
- [Google Scholar]
- Does the utilization of embryo glue give promise in donor embryo fets? Fertil Steril. 2018;109:e32.
- [Google Scholar]
- Hyaluronan-enriched transfer medium improves outcome in patients with multiple embryo transfer failures. J Assist Reprod Genet. 2012;29:679-85.
- [Google Scholar]
- Single fresh and frozen–thawed blastocyst transfer using hyaluronan-rich transfer medium. Reprod Biomed Online. 2007;15:701-7.
- [Google Scholar]
- Effect of embryo glue transfer medium during fresh and frozen-thawed embryo transfer. Fertil Steril. 2014;102:e313.
- [Google Scholar]







