Translate this page into:
To study the role of growth hormone supplementation on endometrial response and implantation rate in frozen thawed embryo transfer (FET) cycles
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aim:
To assess the role of growth hormone in improving endometrial response and clinical outcome.
Materials and Methods:
A prospective randomised study was conducted with 50 patients who were treated with FET cycles at Ridge IVF, Delhi, between August 2019 and January 2020. Patients were classified into two groups: group A administered simultaneous GH along with hormone-replacement therapy (HRT), for endometrial preparation; while group B received HRT alone. GH four IU subcutaneously was started on the day of HRT (day two/three) and continued till the endometrium reached a thickness of 8 mm.
Statistical analysis:
Statistical testing was conducted with the statistical software SPSS 20.0. Continuous variables are presented as mean±SD. Categorical variables are expressed as frequencies and percentages. Categorical data was compared using Chi-square test or Fisher’s exact test as appropriate.
Results:
Patients in group A had a significantly higher endometrial thickness (8.86±1.06 mm) as compared to group B (8.34±1.16 mm) (P value=0.028). However, patients in group A i.e those given GH, had a lower impedance to blood flow as shown by a lower pulsatility index (1.68±0.12 vs 1.92±0.13) (P value=0.028), resistance index (0.73±0.06 vs 0.86±.02) (P value=0.053), and a lower peak systolic velocity/end diastolic velocity of the uterine arcuate artery (2.68±.13 vs 2.93±0.11) (P value=0.066). The clinical pregnancy rate was comparatively more in group A compared to group B (56% vs 48%) (P value=0.588).
Conclusion:
Growth hormone when given along with HRT can improve FET results by enhancing endometrial perfusion.
Keywords
endometrial receptivity
frozen-thawed embryo transfer
growth hormone
INTRODUCTION
Frozen-thawed embryo transfer (FET) is widely used in assisted reproductive technology programs. FET maximizes embryo utilization, effectively preventing complications such as ovarian hyperstimulation syndrome.[1] To further improve FET cycle outcome, efforts have been made to improve techniques for cryopreservation and endometrial preparation.
The addition of growth hormone during ovulation induction could optimize the clinical pregnancy rate by increasing the number of oocytes retrieved and improving the embryo quality.[2,3]
However, limited studies have been conducted to show its effects on endometrial receptivity and implantation.[4,5]
In this study, we aim to evaluate the effects of GH on clinical outcomes after FET.
SUBJECTS AND METHODS
This is a prospective randomized study was conducted over a duration of 6 months (August 2019 to January 2020). The Independent Ethics Committee, Indian Fertility Society, New Delhi (Reg No.ECR/222/Indt/DL2015/RR-2018) approved the research product (Ethical clearance number − IEC/IFS/2020No.52).
Sample size: 50 (sample size of convenience)
Sample size was calculated based on the assumptions that level of significance (α) 5%, power of study 80%, variability, that is, SD and mean difference of total score (on the basis of previous studies).
A sample size of at least 40 cases was needed.
Case (study group): 25
Control group : 25
Complete infertility workup of all patients will be done.
Inclusion criteria:
Age 23 to 38 years.
Freezing of all embryos and FET done in the subsequent cycle.
‘Hormone replacement therapy (HRT) was done for endometrial preparation.’
Embryos cryopreserved by vitrification and a minimum two embryos cryofrozen for each patient.
Exclusion criteria:
Uterine malformations.
Uterine adhesions, submucosal fibroids.
Uncontrolled systemic diseases such as diabetes.
Patients fulfilling the criteria were included in the study. At recruitment, informed consent was taken from every patient.
Patients undergoing FET cycles were randomly allocated to two groups. Randomization was done using a random number table. Group A (study group) patients received 4 IU of GH (eutropin) daily subcutaneously with HRT from day 3 till embryo transfer.
Group B (control group) patients received only HRT from day 3 of cycle. HRT includes administration of Oral estradiol valerate (Tab Progynova) 2 mg TDS per day.
Transvaginal USG was performed to evaluate endometrial thickness (ET).
When ET reached a minimum of 8 mm injection aqua progesterone (aqua susten) 25 mcg subcutaneous was started and given till the day of embryo transfer.
The day progesterone started was considered the ovulation day.
Embryo transfer done on day three embryonal age.
Thawing of embryos was done on day 3 embryonal age and their quality was reviewed. Embryos comprising at least six intact cells and with not more than 20% fragmentation were considered good quality that were then transferred to the uterine cavity under ultrasound guidance. Till 10 weeks, luteal phase support continued with progesterone and estradiol valerate.
Endometrial thickness and morphology, uterine arcuate artery pulsatility index (PI), resistance index (RI), and peak systolic velocity/end-diastolic velocity (S/D) were detected by color Doppler ultrasonography (Voluson P8) with 6 to 10 Mhz multifrequency transvaginal probe on the day of progesterone injection. The endometrial thickness was taken as the maximum thickness between highly reflective interfaces of the endometrial myometrial junction. Endometrial morphology was classified as hyperechoic, isoechoic, and triple-line pattern. The zones of vascular penetration into the subendometrial and endometrial region were described as zone 1 is sub endometrial zone, zone 2 is the outer hyperechogenic zone, and zone 3 is the inner hypoechogenic zone. Blood flow velocity waveforms were obtained by positioning color Doppler window in the thickest area of endometrial–subendometrial area. The PI, RI, and S/D were measured when at least five consecutive stable waveforms were obtained.
A Beta hCG test was taken after 15 days of embryo transfer and confirmed by doubling after 48 hours. If positive, then ultrasound was done after 2 weeks of beta hCG to confirm intrauterine pregnancy and number of gestational sacs.
RESULTS
A total of 50 patients were included in the study, 25 in the study group (group A) and 25 in the control group (group B). The two groups were found to have no difference in terms of basic characteristics (summarized in Table 1)

Of the 50 couples involved in the study, 74% had primary infertility and 26% had secondary infertility. Seventy-two percent of patients in group A suffered from primary infertility, 76% of group B patients had primary infertility as shown in Table 2.

Cause of Infertility
Tables 2-4 describes the distribution of major causes of infertility between the study and the control groups wherein the causes are comparable in terms of nature of the cause of infertility as well as in terms of its prevalence in the subjects from both the groups.


Endometrial thickness was greater in the study group than the control group. The vascularity indices, PI, RI, and S/D ratio, were lower in the study group compared to the control group as shown in the Table 4.
The implantation rate (calculated as the number of gestational sacs seen by the total number of embryos transferred) and clinical pregnancy rate (number of clinical pregnancies by total number of cycles initiated) in the study group were higher than control group but it was not statistically significant [Table 5].

DISCUSSION
This study showed that simultaneous administration of GH with HRT had beneficial effects on endometrial receptivity and eventually the pregnancy rate. Our study is one of the few studies to evaluate both endometrial thickness and vascularity after growth hormone supplementation and to compare both the groups in terms of endometrial thickness and vascularity improvement and clinical pregnancy rates.
The two important indicators of endometrial response that can be evaluated in ultrasound are the thickness of the endometrium and uterine perfusion. Together they can be good tools to evaluate implantation potential.[6,7] In our study, we have taken into account both the parameters, that is, ET and blood flow in uterine spiral arteries. Growth hormone supplementation in the study group shows a significant positive effect on the blood flow indices. The RI and PI were significantly lower in the study group.
Administration of growth hormone showed a beneficial impact on the endometrial thickness in the study group. Studies in the past also have reported a similar positive influence of GH on endometrial thickness as shown in Tables 6-8. One study conducted by Yang et al. documented higher clinical pregnancy and implantation rates without any positive effect on endometrial thickness. This could be explained by the difference in the day of assessing endometrial thickness. The precise mechanism of action of GH on the endometrium in general is unclear. Larger study groups and well-designed RCTs are needed to clarify whether women with thin endometrium benefit from GH supplementation.
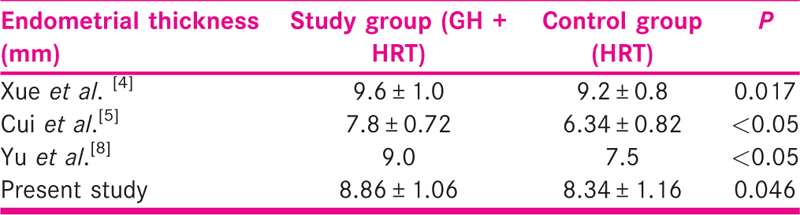
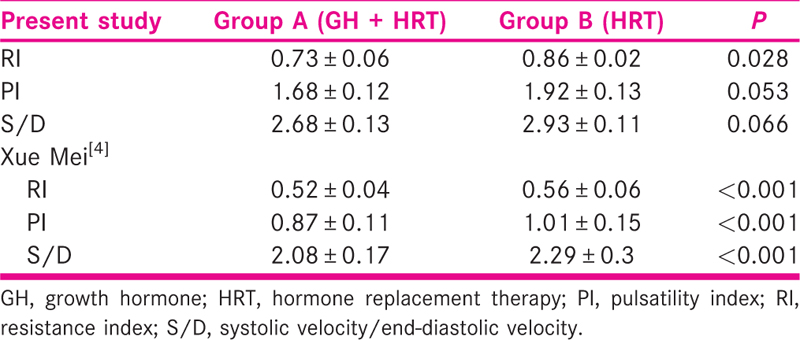

Growth hormone supplementation in the study group shows a significant positive effect on the blood flow indices. The RI and PI were significantly lower in the study group. Very few studies have taken into account both ET and blood flow indices. One such study conducted by Xue-Mei et al. showed better endometrial receptivity and hence better clinical pregnancy rates in the growth hormone group [Table 7].[4]
Successful implantation and establishment of pregnancy require a delicate balance between embryo development and uterine receptivity. Results up to only the first FET are considered in this study. No side effects of growth hormone were seen in the present study.
The dosage and usage of GH vary in different studies. Because of the limited experience with the abovementioned protocols [Table 8] for GH administration, there is a lack of evidence to support the superiority of one method over the other.
Furthermore, the mechanism of GH in the human endometrium remains largely unknown. Because we excluded the impact of embryo quality and maternal age, the underlying biological mechanism seems to be related to endometrial receptivity, which implies that GH might improve the endometrial receptivity through blood flow or at a molecular level. Maternal expression of growth factors and cell adhesion molecules play a major role in the phenomenon of implantation. GH might regulate cytokines produced by the uterus, enhancing endometrial receptivity by controlling the expression of adhesion and anti-adhesion proteins. Cui et al. offered evidence that GH can act in a direct or IGF-1-mediated manner to upregulate receptivity-related molecule expression.[5][6][7][8][9][10]
Further prospective studies and randomized controlled trials would be required in future, for more detailed information about the correlation between GH and endometrium.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- A frozen-thawed embryo transfer program improves the embryo utilization rate. Chin Med J. 2009;122:1974-8.
- [Google Scholar]
- Growth hormone supplementation improves implantation and pregnancy productivity rates for poor-prognosis patients undertaking IVF. Reprod Biomed Online. 2010;21:37-49.
- [Google Scholar]
- Effect of growth hormone on oocyte competence in patients with multiple IVF failures. Reprod Biomed Online. 2009;18:664-70.
- [Google Scholar]
- The effects of growth hormone on clinical outcomes after frozen-thawed embryo transfer. Int J Gynaecol Obstet. 2016;133:347-50.
- [Google Scholar]
- Effects of growth hormone on pregnancy rates of patients with thin endometrium. J Endocrinol Invest. 2019;42:27-35.
- [Google Scholar]
- Ultrasonography and Doppler study to predict uterine receptivity in infertile patients undergoing embryo transfer. J Obstet Gynaecol India. 2016;66(suppl 1):377-82.
- [Google Scholar]
- Role of endometrial blood flow assessment with color Doppler energy in predicting pregnancy outcome of IVF-ET cycles. Reprod Biol Endocrinol. 2010;8:122.
- [Google Scholar]
- Efficacy of growth hormone supplementation with gonadotrophins in vitro fertilization for poor ovarian responders: an updated meta-analysis. Int J Clin Exp Med. 2015;8:4954-67.
- [Google Scholar]
- Effect of growth hormone on the endometrial and endometrial blood flow in frozen thawed embryo tranfer. J Reprod Med. 2013;12:914-7.
- [Google Scholar]
- Influence of growth hormone supplementation in patients with thin endometrium undergoing frozen embryo transfer. Reprod Dev Med. 2019;3:49-53.
- [Google Scholar]







