Translate this page into:
Hypogonadotropic hypogonadism and assisted reproductive techniques: a review
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Backgroud:
Congenital hypogonadotropic hypogonadism (CHH) is a rare genetic disorder that manifests as absent or delayed pubertal development and infertility due to defective secretion or action of gonadotropin-releasing hormone (GnRH). The incidence is 1 in 10,000 in men and 1 in 50,000 in women.
Materials and Methods:
An online search was made on Google scholar and PubMed with search words hypogonadotropic hypogonadism (HHG), in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), intrauterine insemination, male, and female, and the retrospective/prospective studies that met the inclusion criteria were selected.
Inclusion criteria:
The studies included were retrospective and researched the effect of assisted reproductive techniques (ART) on women with CHH. The studies were included if they had any one of the following primary outcomes: fertilization rate (FR), implantation rate (IR), clinical pregnancy rate (PR) per cycle/embryo transfer (ET), and live birth rate (LBR).
Exclusion criteria:
(1) Review articles, (2) case reports, (3) duplication of studies, and (4) studies with no available endpoints. Secondary outcomes were any of the following: abortion rate, multiple gestations, ovarian hyperstimulation syndrome, and any adverse effect. The studies were reviewed for the demographic profile of the patients, drugs, and their doses used for stimulation protocol, fresh/ frozen sample used, ART procedure, number of metaphase II (M II) oocytes retrieved, FR, IR, clinical PR, and adverse outcomes.
Results:
Seven studies have shown a statistically significant increased requirement of dose and duration of gonadotropins in women with CHH while reporting a comparable metaphase II (M II) oocyte recovery rate, FR, PR, IR, and LBR, when compared with controls. Five studies were selected for male HHG with ART, varying from a sample size of 4 to 31. Inj human chorionic gonadotropin (HCG) and Inj human menopausal gonadotropin (HMG)/recombinant follicle-stimulating hormone (rFSH) was used to induce spermatogenesis for a period of 6 to 24 months. In men with azoospermia/unable to conceive after gonadotropin therapy, ICSI was performed. Testicular sperm extraction (TESE) was used for the extraction of sperm in azoospermic men. FR from 41.7% to 82%, CPR from 17.6% to 51.5%, and LBR from 20% to 41.3% have been reported.
Conclusion:
Controlled ovarian hyperstimulation (COH) with IVF/ICSI should be offered to those patients who fail to conceive naturally or with intrauterine insemination/gonadotropin therapy. Newer regimes of COH (HCG with HMG/FSH), vitrified-thawed ET for female HHG, and pretreatment with FSH followed by HCG and follitropins for induction of spermatogenesis in male HHG look promising and need to be researched further.
Keywords
ART
hypogonadotropic hypogonadism
ICSI
infertility male/female
IVF
INTRODUCTION
Congenital hypogonadotropic hypogonadism (CHH) is a rare genetic disorder that manifests as absent or delayed pubertal development and infertility due to defective secretion or action of gonadotropin-releasing hormone (GnRH).[1] It is associated with inappropriately low gonadotropins [luteinizing hormone (LH) and follicle-stimulating hormone (FSH)] and sex steroids (testosterone or estradiol) in the absence of any functional or anatomical defect in the hypothalamic-pituitary gonadal axis (HPG).[2] The incidence is 1 in 10,000 in men and 1 in 50,000 in women.[3] Besides reproductive function, it is also often associated with other abnormalities such as cleft-lip, cleft-palate, deafness, renal abnormalities, neurodevelopmental delay, and cardiac and digital defects.[3,4,5] Hence, it has a negative impact on the patient’s sexual, psychological, bone, and metabolic health.
Etiology
Hypogonadotropic hypogonadism (HHG) may be congenital or acquired. CHH may be broadly divided into anosmic CHH [Kallaman syndrome (KS)] and normosomic congenital HHG (nCHH) [TABLE 1].[2] While traditionally, KS was reported to be more common,[6] recent studies have now reported nCHH as the more common variant.[3,5] With the advent of next-generation massive parallel sequencing (NGS), such as whole-exome sequencing (WES), whole-genome sequencing (WGS), and targeted exome approach, several new genes have been identified.

Genetic origin
Both anosmic and normosmic have a variable pattern of genetic inheritance. Autosomal dominant/recessive, X-linked transmission have been identified. The variable phenotypes can be explained due to the oligogenic inheritance [the phenotype is expressed when there is more than 1 mutant Idiopathic HHG (IHH)/KS gene][6] seen in 10% to 20% cases.
In anosmic variant (KS), there is a defective embryonal migration of GnRH neuron (in association with olfactory receptor neurons) from the neural crest to hypothalamus. Some of the associated gene defects are KAL 1, FGFR1, FGF8, and PROK2.[7] In normosmic variant (nCHH), the mutations can be in the hypothalamic-pituitary region (DAX1, SRA1, etc.), GnRH pulse generator (TAC3, KISS1, KISS1R, GNRH1), pituitary gonadotropes (GNRHR, FSHB, LHB), or when associated with obesity (LEP, LEPR, PC1) and neurodegenerative syndromes [Gorden Holmes Syndrome, 4H syndrome (hypomyelination, HHG, and hypodontia), Martsolf syndrome, and DMXL2].[9]
Pathophysiology
An intact hypothalamic-pituitary-gonadal (HPG) axis is essential for normal pubertal development. The axis is active in utero (and early neonatal life in males), and then becomes dormant, to be reactivated at the time of puberty. The activation of the GnRH induced pulse of LH and FSH leads to the development of pubertal changes. Due to defective GnRH pulse, there is delayed/absent puberty, delayed bone fusion (eunuchoid habitus), and reduced bone mineral density due to decreased sex steroids.
In males, FSH stimulates the proliferation of Sertoli cells and the development of spermatogonia, whereas Leydig cells get stimulated by LH and lead to the production of testosterone. This high concentration of local testosterone leads to spermatogenesis. Due to the lack of HPG axis in utero and the neonatal life, males may present in the neonatal period with cryptorchidism or micropenis.[9,10]
In females, the early stage of follicular growth is independent of these hormones. However, at the time of puberty, LH stimulates theca cells to produce androgens, and FSH is required for final maturation of the follicle, and secretion of estradiol from the granulosa cells. Hence, it often goes unnoticed in females till puberty.
Clinical presentation
Females
Although the primary complaint in females is primary/secondary amenorrhea, a few women have also reported spontaneous menses, and many have shown some development of secondary sexual characteristics [thelarche; pubarche, Tanners stage 3 (36.7%) or 4 (30%); axillary hair growth).[3,5] The other presenting complaints are decreased libido (83.3%), sleep disorder (53.3%), infertility (72.2%), vaginal dryness (40%), and eunuchoid habitus (76.7%).[5]
Males
In males, the chief complaints were micropenis (48.3%) and small testes (37.9%). Other complaints were sleep disorder (75.9%), erectile dysfunction and low libido (72.4%), fatigue (55.2%), high pitched voice (41.4%), and infertility (48%).[5]
CHH may also be associated with metabolic syndromes such as diabetes mellitus and obesity. Patients with KS may present with additional congenital anomalies such as cleft palate, unilateral renal agenesis, split hands, short feet, short metacarpals, deafness, and mirror movements (synkinesia).[9]
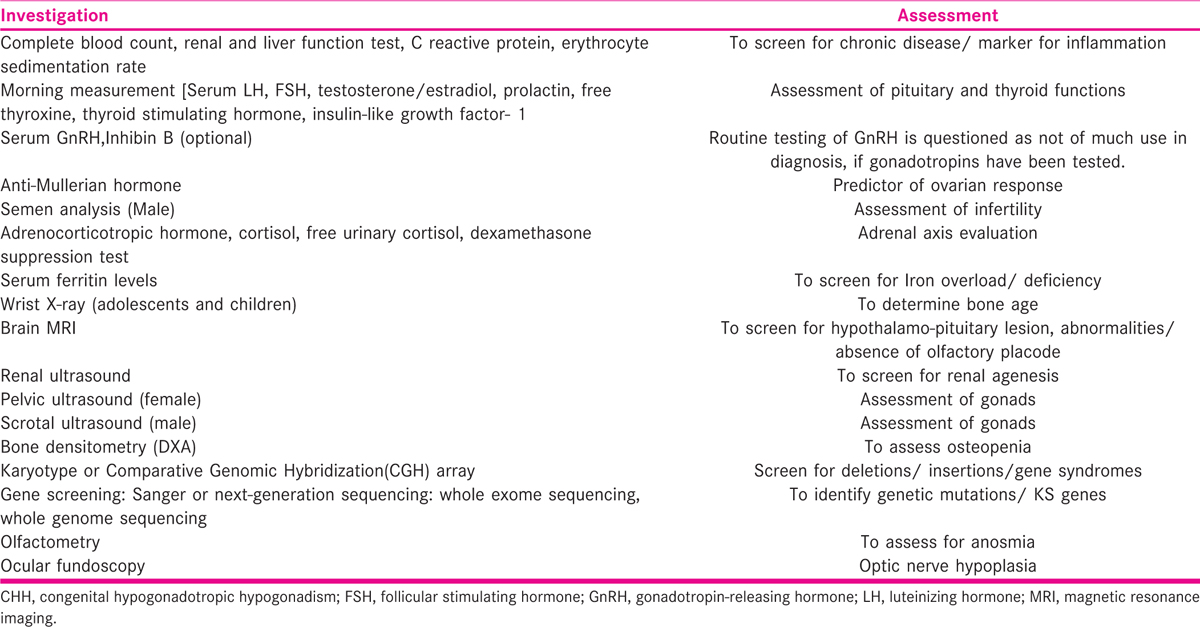
Diagnostic workup
Because CHH is a diagnosis of exclusion, it requires an extensive workup to exclude acquired causes of HHG and also to screen for associated defects and genetic mutations [TABLE 2]. Many patients present with infertility and hence, need to be evaluated for the same. Although semen analysis, gonadal ultrasound, and hormonal profile are done for the affected males, the affected women undergo tests for ovarian reserve, besides the hormonal profile and the complete infertility workup.
Antral follicle count (AFC) may be difficult to interpret in these women, due to the smaller size of the ovaries and low pool of antral follicles. Because of ovarian follicles up to 4 mm diameter act as the main source of serum anti-Mullerian hormone (AMH). AMH may be used to predict ovarian response and tailor the dose of gonadotropins for assisted reproductive techniques (ART) cycles in these women.[10,11]
Treatment
The treatment in adults is directed to the desire of the patient, whether they want fertility or not. In case they do not wish to conceive, they can be put on hormone replacement therapy (testosterone for the male, estrogen, and progesterone for the female). This will help improve bone mineral density, epiphyseal closure (young adults), development of secondary sexual characteristics, improved libido, and help to restore self-confidence and a sense of wellbeing. In females, estrogen is started in a low dose of 1 to 2 mg/day, and gradually increased over a period of 12 to 24 months, to allow the development of secondary sexual characteristics. Then the patient is put on cyclic progesterone (200 mg for 14 days) to prevent endometrial hyperplasia. In men, testosterone enanthate 200 mg once a month then 2 to 3 weekly may be given. Lifelong therapy is required as the reversal is observed in only 10% to 20% of the cases.[8]
Genetic counseling
Genetic counseling should be initiated at the first visit itself. Counseling should be done regarding the phenotypic expression of the identified gene defects, for example, the possibility of renal agenesis if the mutation lies in ANOS1/KAL1 or primary adrenal failure and infertility if there is DAX1/NROB1 mutation. It is also important to counsel the patient regarding the risk of inheritance (Mendelian or oligogenic) and its expected phenotypic expression, when they seek treatment for infertility in adult life. For example, in the pedigrees indicating autosomal dominant transmission, whereas the risk of transmission to the offspring is 50%, the phenotypic expression is variable and difficult to predict.[6]
Treatment for infertility
A literature search for the available treatment options for a patient with HHG was made.
MATERIALS AND METHODS
An online search was made on Google scholar and Pubmed with search words “hypogonadotropic hypogonadism,” “IVF,” “ICSI,” “IUI,” “male,” and “female,” and the retrospective/prospective studies that met the inclusion criteria were selected.
Inclusion criteria
The studies included were retrospective and researched the effect of ART on women with CHH as there were no prospective studies. Studies were included if they had any one of the following primary outcomes: fertilization rate (FR), implantation rate (IR), clinical pregnancy rate (PR) per cycle/embryo transfer (ET), and live birth rate LBR.
Secondary outcomes were any of the following: abortion rate, multiple gestations, ovarian hyperstimulation syndrome, and any adverse effect.
Exclusion criteria
(1) Review articles, (2) case reports, (3) duplication of studies, and (4) studies with no available endpoints.
The studies were reviewed for the demographic profile of the patients, drugs and their doses used for stimulation protocol, fresh/frozen sample used, ART procedure, number of metaphase II (M II) oocytes retrieved, FR, IR, clinical PR, and adverse outcomes.
RESULTS
Nine studies were selected on ART in female HHG [TABLE 3], all were retrospective, with a sample size varying from 7 to 81. Seven of nine studies had a well-matched control group of either tubal factor, mild male factor, or unexplained infertility.
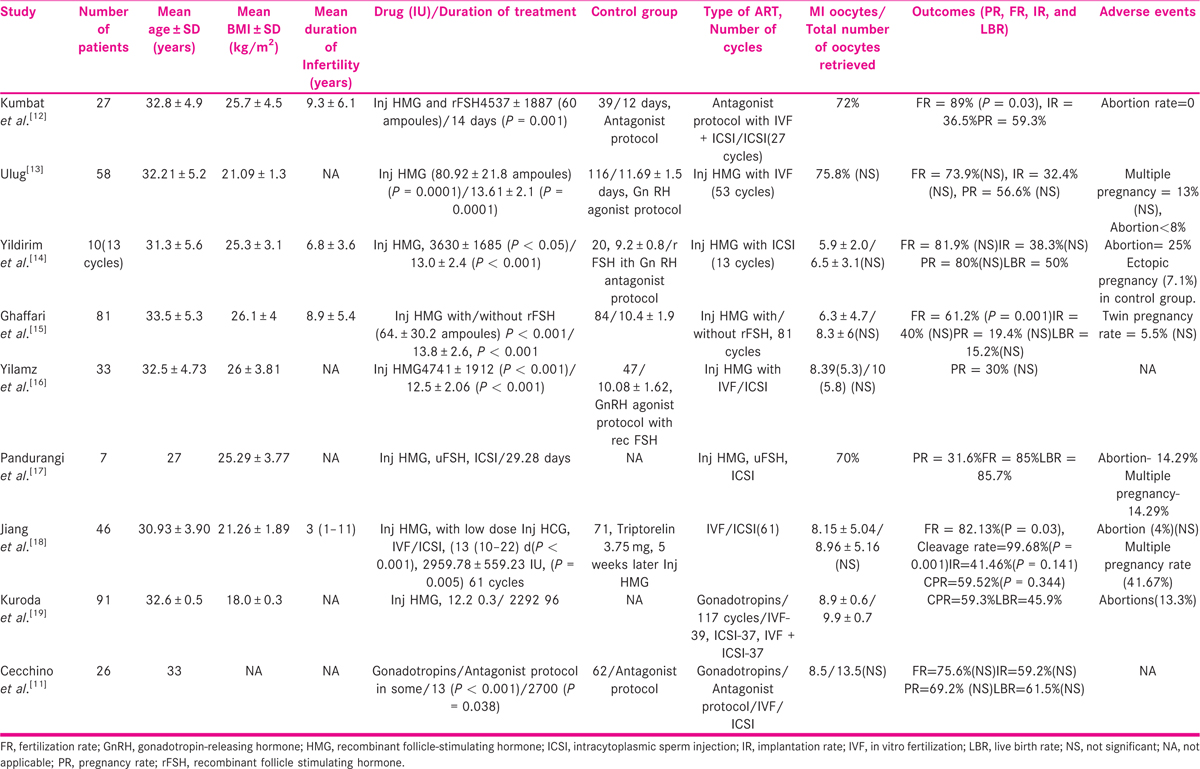
Most studies (six) have used human menopausal gonadotropin (HMG), supplementing it with either urinary/recombinant follicle-stimulating hormone (rFSH; two). Cechinno et al.[11] have used Luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and have reported a significantly higher dose of LH required in women with HHG.
Seven studies have shown a statistically significant increased requirement of dose and duration of gonadotropins in women with CHH, whereas reporting a comparable M II oocyte recovery rate, FR, PR, IR, and LBR, when compared with controls. However, Gaffari et al.[15] reported a statistically significant difference in the fertilization rate, but no difference in PR and LBR, when matched with controls.
Five studies were selected for male HHG with ART, varying from a sample size of 4 to 31 [TABLE 4]. None of them had a control group. Inj human chorionic gonadotropin (HCG) and Inj HMG/rFSH was used to induce spermatogenesis for a period of 6 to 24 months. In men with azoospermia/unable to conceive after gonadotropin therapy, intracytoplasmic sperm injection (ICSI) was performed. Testicular sperm extraction (TESE) was used for the extraction of sperm in azoospermic men. FR from 41.7% to 82%, CPR from 17.6% to 51.5%, and LBR from 20% to 41.3% have been reported.
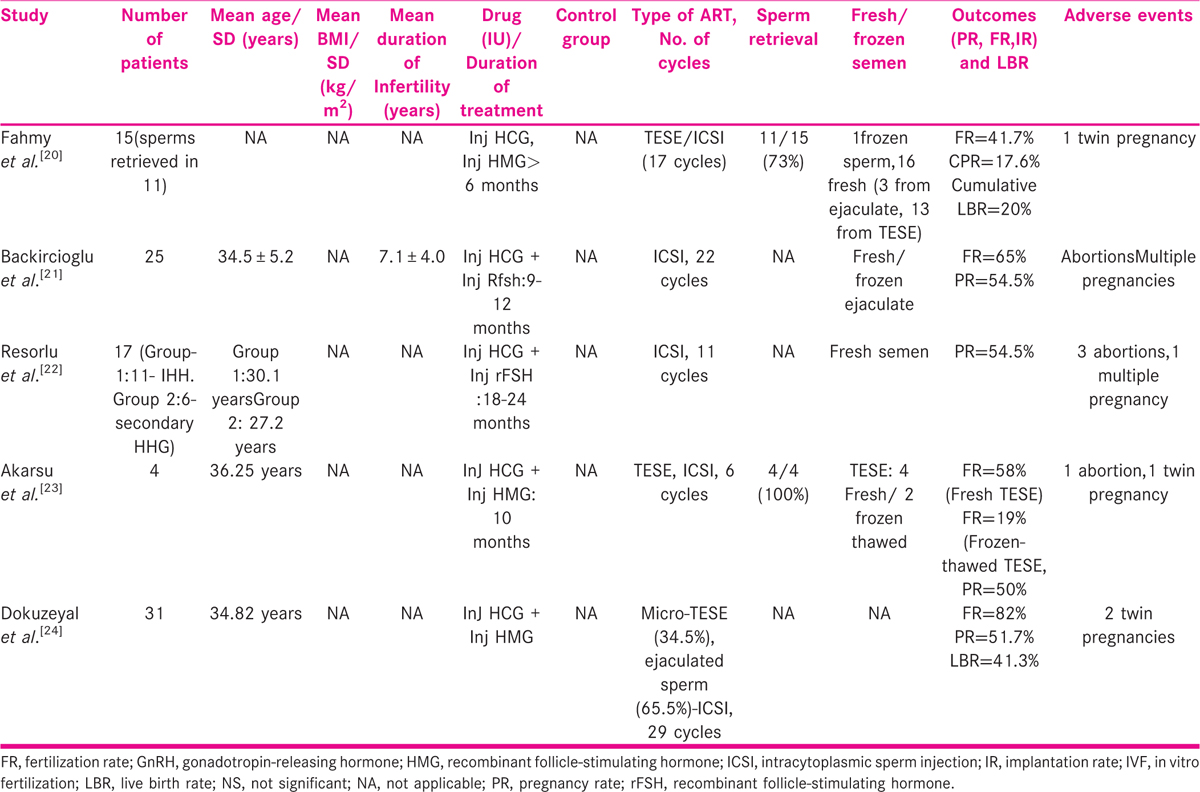
Adverse events
Six studies commented on adverse events. Eight reported abortions and nine multiple pregnancies. None of the studies reported ovarian hyperstimulation syndrome (OHSS).
DISCUSSION
HHG is a rare cause of infertility (1%) hence, there were no prospective trials.[12] The studies available are few, have a small sample size, heterogeneous, and retrospective in design, which makes them prone to selection bias.
The women in these studies have been pretreated with combined estrogen and progesterone for 2 to 3 months before commencing ART cycle. The uterus is hypoplastic in these women, and it helps to regenerate the endometrium and increase the size of the uterus. Most of the studies (eight) have used HMG or combined FSH and LH for controlled ovarian hyperstimulation (COH) in women with CHH without pituitary suppression. Fig. 1
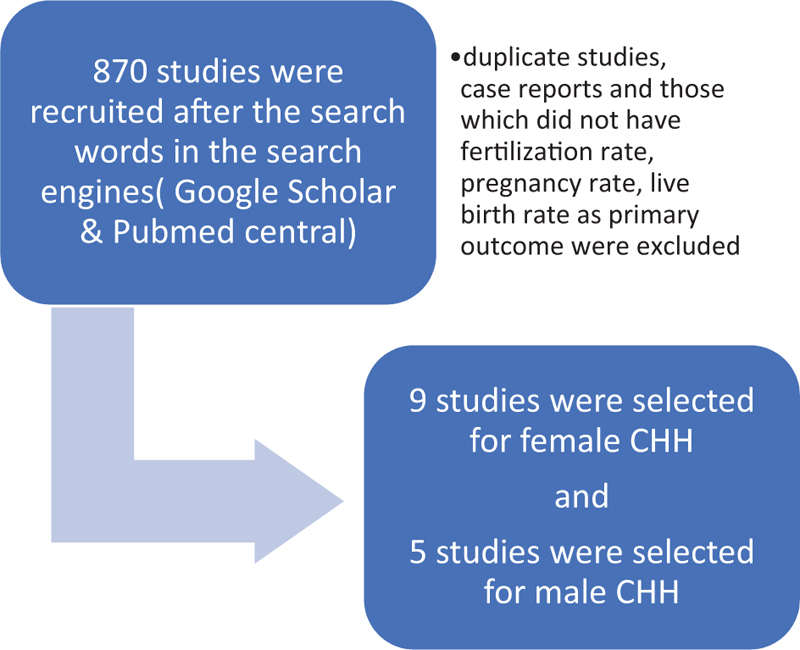
- Selection of studies
LH is required for androgen production from theca cells, which acts as a substrate for the aromatase enzyme and gets converted to estrogen by the granulosa cells. Although early ovarian follicles have receptors for FSH only, as they mature, they express receptors for both LH and FSH and get responsive to both. LH completes the meiotic division in the oocyte, releases oocytes, and causes the development of corpus luteum. Although FSH may be sufficient in eugonadotropic women, women with hypogonadotropic hypogonadism need either inj HMG or combined recombinant FSH and LH.[12]
A new protocol of low dose HCG (80–300 units) combined with HMG has been suggested by Jiang et al.[18] for COH in in vitro fertilization (IVF) cycles for women with HHG harvesting a comparable number of embryos without affecting embryo quality. It offers the added advantage of reduced duration of HMG use, making the cycle more convenient and cost-effective.
Effect of age
Gaffari et al.[15] reported a higher requirement of gonadotropins for women older than 35 years, with a lower recovery of total and M II oocytes, without affecting their FRs, IRs, PRs, and LBRs. However, older women had significantly lower PRs/cycle (P = 0.01). Yilmaz et al.[16] observed age to be a significant marker for ovarian response in women with HHG.
Frozen embryo transfer versus fresh cycle
Jiang et al.[18] have reported no statistical difference in the biochemical PR, clinical PR, multiple PR, ongoing PR per transfer, and IR after ET in frozen ET cycles and fresh cycles.
Kuroda et al.[20] reported outcome of 135 single ET after vitrified-warmed cleavage/blastocyst and the CPR and LBR were 34.6%, 26.9%, 65.1%, and 50.5%, respectively. Because COH in women with CHH requires prolonged and high doses of gonadotropins, it can cause embryo-endometrial asynchrony, a higher chance of multiple pregnancies, and OHSS. There was no case of multiple pregnancies or OHSS reported in the study. Hence, segmented cycles with single ET were suggested to improve ART outcomes.
Males
GnRH pulsatile therapy can induce spermatogenesis, but is usually not preferred, due to limited availability and requirement of infusion pumps. Conventionally, a trial of HCG for 3000 to 5000 IU/week for 6 months followed by HMG/FSH (75 IU three times per week) is prescribed. Both the drugs can be given together to induce spermatogenesis in men with small testicular volume (<4 ml) or with history of orchidopexy. A new approach is to pretreat the men with FSH to prime the Sertoli cells and seminiferous tubules, followed by administration of HCG, to prevent premature differentiation of Sertoli cells under the influence of LH-induced intratesticular testosterone. However, It may take around 6 months to 2 years to affect spermatogenesis with gonadotropins/HCG. The results may not be achieved in many (testicular volume < 4ml, h/o orchidopexy).[25]
Due to the prolonged course of treatment, and uncertain results, IVF or ICSI with TESE for testicular sperm extraction/semen is recommended if the initial trial with FSH/HCG fails to affect spermatogenesis, in order to shorten the treatment to pregnancy interval.
CONCLUSION
COH with IVF/ICSI should be offered to those patients who fail to conceive naturally or with intrauterine insemination/gonadotropin therapy. Newer regimes of COH (HCG with HMG/FSH), vitrified-thawed embryo transfer for female HHG, pretreatment with FSH followed by HCG, and follitropins for induction of spermatogenesis in male HHG look promising and need to be researched further.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Genetic testing facilitates prepubertal diagnosis of congenital hypogonadotropic hypogonadism. Clin Genet. 2017;92:213-16.
- [Google Scholar]
- Central hypogonadotropic hypogonadism: genetic complexity of a complex disease. Int J Endocrinol. 2014;2014:1-13.
- [Google Scholar]
- Clinical characteristics of 138 Chinese female patients with idiopathic hypogonadotropic hypogonadism. Endocr Connect. 2017;6:800-10.
- [Google Scholar]
- Phenotypic spectrum and hormonal profile in hypogonadotropic hypogonadism. J Med Life. 2014;7:42-45.
- [Google Scholar]
- The clinical and psychological profiles of patients with hypogonadism, followed in 3 reference hospitals of Cameroon: an observational study. Pan Afr Med J. 2019;33:47.
- [Google Scholar]
- Genetics in endocrinology: genetic counseling for congenital hypogonadotropic hypogonadism and Kallmann syndrome: new challenges in the era of oligogenism and next-generation sequencing. Eur J Endocrinol. 2018;178:R55-R80.
- [Google Scholar]
- The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol. 2009;5:569-76.
- [Google Scholar]
- Expert consensus document: European consensus statement on congenital hypogonadotropic hypogonadism–pathogenesis, diagnosis, and treatment. Nat Rev Endocrinol. 2015;11:547-64.
- [Google Scholar]
- Update on the genetics of idiopathic hypogonadotropic hypogonadism. J Clin Res Pediatr Endocrinol. 2017;9(suppl 2):113-22.
- [Google Scholar]
- Serum anti-Mullerian hormone levels correlate with ovarian response in idiopathic hypogonadotropic hypogonadism. J Assist Reprod Genet. 2012;29:597-602.
- [Google Scholar]
- Impact of hypogonadotropic hypogonadism on ovarian reserve and response. J Assist Reprod Genet. 2019;36:2379-84.
- [Google Scholar]
- Women with hypogonadotropic hypogonadism: cycle characteristics and results of assisted reproductive techniques. Acta Obstet Gynecol Scand. 2006;85:1453-7.
- [Google Scholar]
- The reproductive performance of women with hypogonadotropic hypogonadism in an in vitro fertilization and embryo transfer program. J Assist Reprod Genet. 2005;22:167-71.
- [Google Scholar]
- Comparison of reproductive outcome of the women with hypogonadotropic hypogonadism and tubal factor infertility. Clin Exp Obstet Gynecol. 2010;37:120-2.
- [Google Scholar]
- Assisted reproductive technique outcomes in hypogonadotropic hypogonadism women. Ann Saudi Med. 2013;33:235-40.
- [Google Scholar]
- The reproductive outcome of women with hypogonadotropic hypogonadism undergoing in vitro fertilization. Syst Biol Reprod Med. 2015;61:4. 228-32
- [Google Scholar]
- Pregnancy outcome of assisted reproductive technology cycle in patients with hypogonadotropic hypogonadism. J Hum Reprod Sci. 2015;8:146-150.
- [Google Scholar]
- The effects of low-dose human chorionic gonadotropin combined with human menopausal gonadotropin protocol on women with hypogonadotropic hypogonadism undergoing ovarian stimulation for in vitro fertilization. Clin Endocrinol. 2018;88:77-87.
- [Google Scholar]
- Infertility treatment strategy involving combined freeze-all embryos and single vitrified-warmed embryo transfer during hormonal replacement cycle for in vitro fertilization of women with hypogonadotropic hypogonadism. J Obstet Gynaecol Res. 2018;44:922-28.
- [Google Scholar]
- ICSI using testicular sperm in male hypogonadotropic hypogonadism unresponsive to gonadotropin therapy. Hum Reprod. 2004;19:1558-61.
- [Google Scholar]
- MicroTESE and ICSI outcomes of azoospermic men with hypogonadotropic hypogonadism after one year combined HCG and FSH treatment. Hum Reprod. https://doi.org/10.1093/humrep/27.S2.73.
- [Google Scholar]
- Is intracytoplasmic sperm injection essential for the treatment of hypogonadotrophic hypogonadism? A comparison between idiopathic and secondary hypogonadotropic hypogonadism. Hum Fertil. 2009;12:204-8.
- [Google Scholar]
- Pregnancies achieved by testicular sperm recovery in male hypogonadotrophic hypogonadism with persistent azoospermia. Reprod Biomed Online. 2009;18:455-9.
- [Google Scholar]
- Evaluation of intracytoplasmic sperm injection outcomes in men with hypogonadotropic hypogonadism: 5 years’ experience. Fertil Steril. 2010;94:S16.
- [Google Scholar]
- Managing congenital hypogonadotropic hypogonadism: a contemporary approach directed at optimizing fertility and long-term outcomes in males. Ther Adv Endocrinol Metabol. 2019;10:1-15.
- [Google Scholar]







