Translate this page into:
A prospective randomized comparative study between transdermal estradiol gel and oral estradiol valerate tablets for successful clinical outcome in frozen-thawed embryo transfer cycles
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
The aim of this study was to evaluate the use of transdermal estradiol gel and compare it with oral estradiol valerate tablets for the preparation of endometrium in frozen-thawed embryo transfer (FET) cycles.
Methods:
This prospective trial included 100 women undergoing FET cycles during study period and they were randomized into one of the two groups. Group 1 (n = 50) received oral estradiol valerate tablet and group 2 (n = 50) received transdermal estradiol gel from day 2 of menstrual cycle and endometrial thickness monitored with transvaginal ultrasonography. Primary outcome of this study was to compare clinical pregnancy rate (CPR) between the two groups. Secondary outcomes were implantation rates (IRs), CPRs, miscarriage rates (MRs), endometrial thickness at the start of progesterone, cycle cancellation rates, undesirable side effects, and patient satisfaction score. Statistical testing was performed with SPSS 17.0.
Results:
There was no clinically significant difference in biochemical pregnancy rate, CPR, IR, and MR between the two groups. Endometrial thickness on day of progesterone start was higher in group 2 as opposed to group 1 (9.81 ± 0.861 vs. 9.46 ± 0.830; P-value = 0.043) which was clinically significant. Almost 37.5% patients (n = 18) in group 1 had mild adverse effects when compared with only 12.76% (n=6) in gel group (group 2).
Conclusion:
We conclude that transdermal estradiol gel is equally efficacious as oral estrogen in hormone replacement FET cycles but with added advantage of better patient comfort and lesser side effects with transdermal gel.
Keywords
Estradiol valerate
FET cycle
HR-FET
transdermal estradiol
INTRODUCTION
With the recent advances in the field of cryobiology, it has become possible to reduce complications associated with artificial reproductive technologies such as ovarian hyperstimulation syndrome and adverse perinatal outcomes associated with multiple pregnancy by transferring lesser number of embryos and freezing the rest without compromising the results.[1,2,3] Also it has proved very useful in patients with high progesterone in early follicular phase (>1.5 ng/ml); in patients with poor ovarian function, in whom embryo pooling is performed, in patients in whom egg or embryo donation is used.[4,5] These advances have lead to dramatic increase in frozen-thawed embryo transfer (FET) cycles.[6,7,8] For success of FET cycles, synchronization between embryo and endometrium is vital and the embryo should be transferred during the window of endometrial receptivity [Window of implantation (WOI)-short period of 3–5 days, usually day 18 to day 23].[8,9,10,11,12,13,14]
There are mainly three protocols to prepare endometrium for this purpose: (1) natural cycle FET (spontaneous cycle FET) in an ovulatory patient and modified natural cycle FET. In both these cycles, embryo transfer is performed 3 to 5 days after ovulation depending on when embryo was frozen. (2) Stimulated cycle FET: in which mild exogenous ovarian stimulation is performed with clomiphene citrate (CC), aromatase inhibitors (Ai), gonadotropins, CC or AI + gonadotropins, gonadotrophin-releasing hormone analog/agonist (GnRHa) with human chorionic gonadotropin (hCG) trigger with or without luteal-phase support (LPS) to increase serum estrogen levels leading to enhanced endometrial receptivity. It requires intense monitoring and is not recommended now a days. (3) Artificial cycle/hormone replacement (HR) with or without GnRHa downregulation of hypothalamo-pituitary axis. Here artificial preparation of the endometrium is performed through exogenous estrogen and progesterone with or without the pretreatment with a gonadotropin-releasing hormone agonist. It has been shown that cycles in which GnRHa suppression is not used, they are also equally successful.[15] If GnRHa is not used, the downregulation of hypothalamus axis is achieved by exogenous administration of 17ß-estradiol and progesterone. This is quite commonly used method.
In an artificial cycle to mimic the endocrine conditions of the endometrium of a normal cycle, estrogen and progesterone are administered consecutively and are the two main hormones used for endometrial preparation with or without GnRHa suppression. Estrogen administration is begins at the start of the cycle, causing endometrial development when suppressing dominant follicle development. To avoid risk of unwanted ovarian follicular development and ovulation, estrogen administration should be started before day 4, the earlier the better. This estradiol priming has been shown to cause proliferation of basal cells in endometrium and causes the induction of appropriate progesterone receptors to induce endometrial receptivity.[16,17] Once 7 to 8 mm endometrial development is observed using transvaginal ultrasound monitoring; progesterone administration is started to start secretory changes and is considered day 0 of progesterone administration. On day 3/5 embryo transfer is performed according to stage of embryo (8-cell stage/blastocyst stage).
Estrogen can be administered orally or parenterally.[18] The oral route of estrogen is easy to use, well-tolerated with good patient compliance[17] but it undergoes extensive first pass metabolism leading to systemic side effects such as gastrointestinal upset, increased risk of thromboembolism especially in predisposed group.[17,18,19,20] The first-pass hepatic metabolism can be avoided by using parenteral routes of transdermal, intramuscular (IM), or vaginal type.[17,18] The transdermal route is easy to use and with much less side effects[21] and more physiologic E1/E2 ratio (1–1.5).[17,22] Other parenteral routes are intravenous, IM, and vaginal route. These routes are not usually used for this purpose as mostly invasive and uncomfortable.
The aim of this prospective randomized clinical trial was to compare the efficacy of two methods of endometrial preparation for HR-FET cycles; 17β-estradiol transdermal gel and oral estradiol valerate tablets for successful clinical outcomes.
MATERIALS AND METHODS
This prospective randomized controlled comparative study included a total number of 100 women who were to undergo either conventional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) for FET cycle due to any reason at Akanksha IVF Center from November 2020 to April 2021.
After enrollment in the study, patients underwent full clinical history taking, physical examination, complete hematological and biochemical screening which included complete blood count, blood sugar, urine routine and microscopy, thyroid-stimulating hormone, prolactin, human immunodeficiency virus, Australia antigen (HbsAg), venereal disease research laboratory (VDRL), and anti-hepatitis C virus to look for inclusion and exclusion criteria.
Inclusion criteria were: Patients 21 to 39 years of age who underwent IVF/ICSI and cryopreserved their embryos, a normal uterine cavity assessed by 2D ultrasonogram (USG)/hysteroscopy, HR-FET cycles, transfers involving at least two good quality embryos [day 3; grade 1 or 2 Istanbul consensus 2011(42)].
Exclusion criteria were : uterine anomalies, known case of severe adenomyosis and severe endometriosis, fibroid uterus, endometrial polyp, known case of Asherman syndrome/endometrial tuberculosis, underlying disorders (cardiac/renal/hepatic/thromboembolic disorders/history of depression, etc.), bad quality embryos.
These inclusion and exclusion criteria were included to eliminate uterine and embryonic factors which can influence the success of a cycle and to optimize our results. All the women underwent either conventional IVF or ICSI. Also, embryo cryopreservations were carried out. In the embryo freezing and thawing protocols, embryo transfer catheters (soft catheter) used for embryo transfer were the same.
After ethical clearance and proper informed consent patients were enrolled into one of the two groups randomly by a computer-generated randomization program. Group 1 received 6 mg/day estradiol valerate (E2) oral tablets (2 mg thrice daily) and group 2 received 1.25 g transdermal estradiol gel (17β-estradiol 0.06% gel) (one actuation = 1.25 g gel = 0.75 mg of estradiol) thrice daily without GnRHa suppression. In both the groups, medication was started on day 2 of menstrual cycle and endometrial thickness was monitored from day 2 after ascertaining ovarian suppression by baseline transvaginal sonography. On day 7 of menstrual cycle, dose of estrogen was increased to 4 mg twice daily (total 8 mg) for oral estradiol valerate tablet group (group 1) and two actuations of transdermal estradiol gel twice daily (group 2).
On the 11th day of menstrual cycle, the third ultrasound was performed and the thickness of endometrium was estimated again and again. Estrogen doses were increased 4 mg thrice daily in oral estradiol valerate tablet group (group 1) and two actuations thrice daily in transdermal estradiol gel group (group 2). On day 14, patients were called again for USG follow-up, and if endometrial thickness was more than 7 mm, based on embryo’s age, progesterone in oil was started intramuscularly (100 mg) once daily for 4 days before embryo transfer. The embryo transfers were performed in operation room with soft embryo transfer catheter under 2D-ultrasound guidance in sterile conditions. All transfers were carried out by a single clinician to avoid bias. Two embryos were transferred after thawing as day 3 embryos and then culturing for 1 day till compaction stage. On the day of progesterone start, time taken to reach 7 mm thickness was also noted.
If even by day 15, the endometrial thickness remains <7 mm, then the cycle was cancelled and embryo transfer was not carried out. After embryo transfer, while estrogen administration was continued either by oral or transdermal route, for LPS, patients received a once daily dose of IM progesterone in oil 100 mg once daily for LPS. After 14 days of embryo transfer, urine pregnancy test was performed, and if positive, it was followed by serum β-hCG assay. If pregnancy occurred, the same daily dose of estrogen was continued till 10th week and was tapered off before stopping, and progesterone was continued at the same daily dose until the 12th week of gestation. At 7 weeks, a transvaginal ultrasound was performed to monitor early pregnancy and for cardiac activity. Patients were asked to rate their satisfaction of the treatment and also they were asked if they had any side effects such as nausea, vomiting, erythema, itching at the site of gel application, etc., at the end of treatment.
Primary outcome of this study was to compare clinical pregnancy rate (CPR) between the two groups. Secondary outcomes included implantation rates (IRs), CPR, miscarriage rates (MRs), duration of estrogen administration, endometrial thickness at the start of progesterone, cycle cancellation rates, undesirable side effects between both the groups, and patient satisfaction score.
Biochemical pregnancy rate was defined as a serum β-hCG level >25 IU/l 14 days after embryo transfer. CPR was taken as the presence of a gestational sac with heart beat identified by vaginal/abdominal USG at 7 weeks period of gestation. IR was determined as the ratio of gestational sacs to the number of embryos transferred and miscarriage was regarded as pregnancy loss before 12 weeks of gestation. Cycle cancellation was defined as a cycle abandoned before the embryo transfer OR if ET remains <7 mm even by day 15 of estrogen administration.
Ethical consideration
The study was approved by the Ethics Committee Review Board of Indian Fertility Society. The written informed consent was obtained from all participants after giving them all the needed information.
Statistical analysis
Statistical testing was conducted with the statistical package for the social science system version SPSS 17.0 [International business machines (IBM)]. For all statistical tests, a P-value <0.05 was taken to indicate a significant difference.
RESULTS
Figure 1 shows consort flow chart, which shows a total of 100 patients who were undergoing FET cycles during the study period were recruited for this study, and were randomized into one of the two groups according to inclusion and exclusion criteria. Group 1 received oral estrogen tablets (oral group) and group 2 received transdermal estradiol gel (gel group) for endometrial preparation. In group 1 (oral tablet group), two cycles were cancelled and in group 2 (gel group), three cycles were cancelled.

- Consort flow chart.
Basic and demographic characteristics are summarized in Table 1. Both the groups were comparable in terms of age, body mass index (BMI), duration of infertility, type and cause of infertility, duration of infertility, cause of FET cycle. The cause of infertility was not significantly different between the two groups. The cause of infertility was female factor only in 35.41% (n = 17), male factor only in 31.25% (n = 15), combined factor in 12.5% (n = 6), and unexplained in 20.83% (n = 10) of patients in group 1. The cause of infertility was female factor only in 38.29% (n = 18), male factor only in 31.91% (n = 15), combined factor in 19.14% (n = 9), and unexplained in 10.63% (n = 5) patients in group 2. In group 1, 64.58% had primary infertility and 35.41% secondary infertility. In group 2, 61.70% had primary infertility and 38.29% had secondary infertility. About 16.66% women had history of IVF success and 31.25% women had history of IVF failure previously in group 1, whereas 14.89% women had history of IVF success and 31.91% women had history of IVF failure previously in group 2 and this was not significantly different between the two groups.

Table 2 summarizes outcome characteristics. Both biochemical pregnancy rate and CPR were higher in gel group (group 2) than in oral group (group 1); 57.44% versus 56.25% (P-value = 0.906) and 53.19% versus 52.08% (P-value = 0.913) but it was not clinically significant. MR was lower in gel group, 8% in group 2 versus 16% in group 1 (P–value = 0.385), but it did not reach clinical significance. IR was higher in group 2 (34% versus 32.29%, P-value = 0.797), but this was not clinically significant. Endometrial thickness on day of progesterone start was higher in group 2 as opposed to group 1 (9.81 ± 0.861 vs. 9.46 ± 0.830; P-value = 0.0428) which was clinically significant. Almost 37.5% patients (n = 18) in group 1 had mild adverse effects (breast discomfort, nausea, gastric upset, and headache) when compared with only 12.76% (n = 6) in gel group (group 2) and this was clinically significant (P-value = 0.005). There were no cases of ectopic pregnancy in any of the groups.
Patient satisfaction score
Patients were asked to rate their satisfaction of the treatment they received at the end of their treatment on a scale of 0 to 10, where 0 is patient not at all satisfied with the treatment and 10 is patients were totally satisfied with the treatment. Overall patient satisfaction was significantly higher with gel group (8.09 ± 0.97 vs. 7.04 ± 1.05 and P-value < 0.01).
Table 3 summarizes the cycle cancellation in both the groups. In group 1 (oral tablet group), two cycles were cancelled: one due to thin endometrium and the other due to thin endometrium and bleeding per vaginum. In group 2 (gel group), three cycles were cancelled: two due to thin endometrium and one due to fever.

Figure 2 shows comparison of clinical and biochemical pregnancy rates in both the groups.
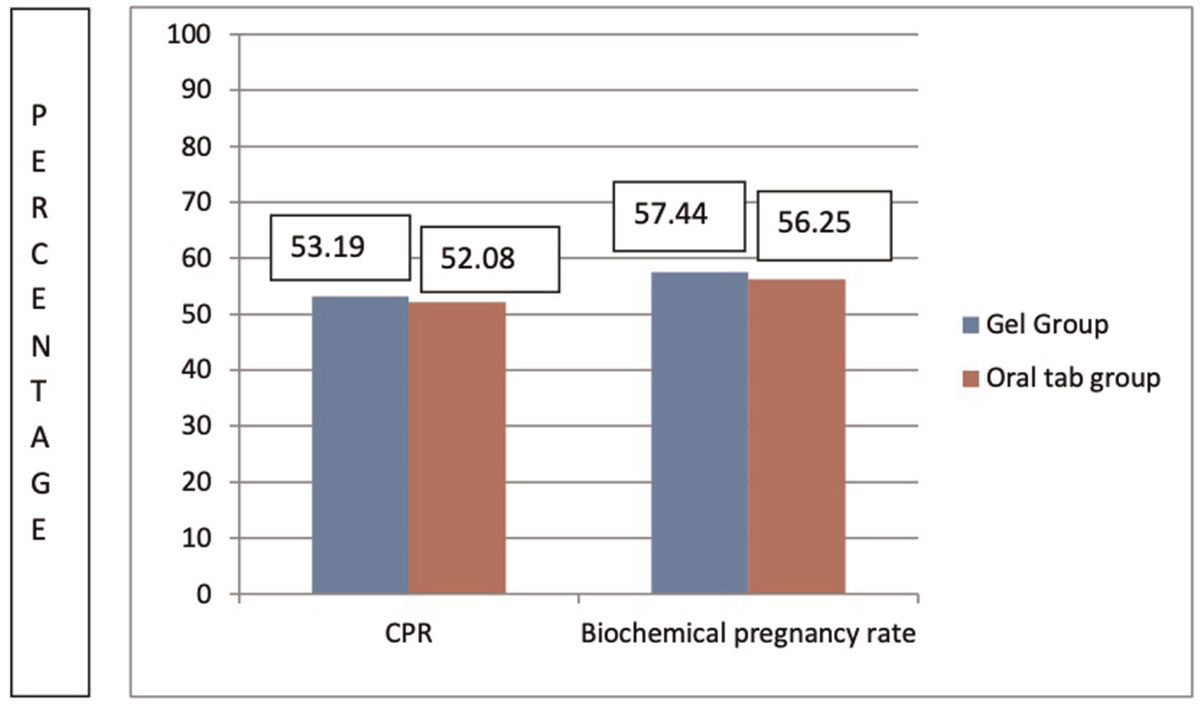
- Clinical and biochemical pregnancy rates in the two groups.
Figure 3 shows comparison of miscarriage rates and adverse effects between both the groups.
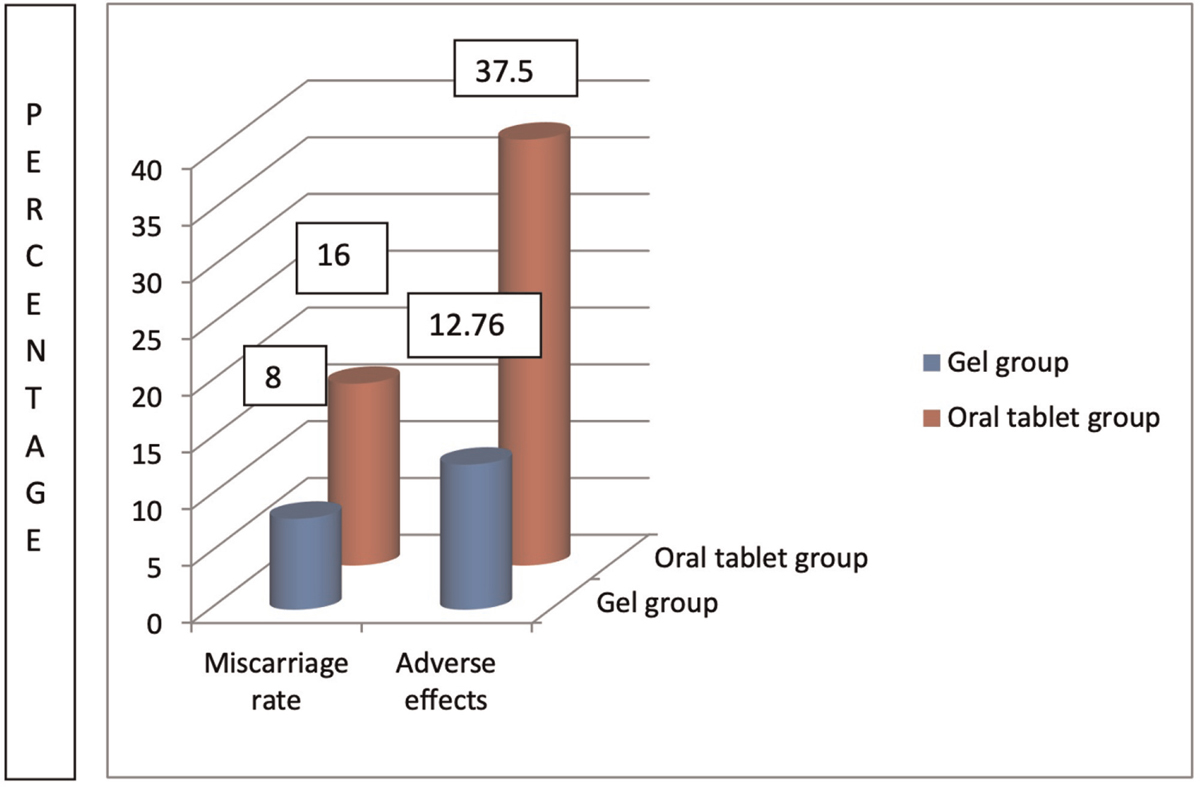
- Miscarriage rates and adverse effects in the two groups.
DISCUSSION
Estrogen given orally undergoes extensive first pass metabolism in intestines and liver and gets converted into estrone and estrone sulfate which are its nonphysiologic metabolites. This leads to steady-state estrone levels 3 to 6 times higher than estradiol,[17] as also shown by Burks and Paulson[20] in a literature review carried out in 2015. These metabolites get concentrated in liver and it is well-known that this supraphysiologic serum levels of estrone can by themselves induce metabolic and enzymatic changes in liver.[23,24] The main two metabolites of oral estrogen estrone and estrone sulfate remain in high concentrations and from this reservoir, E2 is delivered, maintaining high estrogen concentration which is the reason for higher risk of undesirable side effects such as affecting the liver proteins like renin substrate/angiotensinogen, altering sex hormone-binding globulin, thyroxin-binding globulin, corticosteroid-binding globulin, clotting factors and lipid profile leading to hypertension, intravascular clotting, and venous thromboembolism.[17] Also oral estrogen is metabolized into folliculin in liver[23] and intestines leading to induction of the production of coagulation proteins, and can also cause breast hyperplasia and proto-oncogene expression. Absorption of oral estrogen from intestine differs between different individuals and also due to first pass metabolism levels may fluctuate causing breakthrough bleeding in few cases. Orally given estrogen has 3% to 5% bioavailability in comparison to transdermal route which has up to 10% bioavailability, thus requiring lower dose of estrogen for same effect.[23,24]
With transdermal route, amount of conversion to E1 of the absorbed E2 is significantly less, causing higher estradiol valerate concentrations than those of estrone; with E2/E1 ratio of about 1 to 1.5 which is more physiologic,[17,22] while with oral administration this ratio (estradiol/estrone) is 0.2 or E1/E2 ratio 5:1.[22]
Our study shows no clinically significant differences in CPR, biochemical pregnancy rate, and MR between the two methods of endometrial preparation using transdermal estradiol gel and oral estradiol tablets, but better endometrial thickness with transdermal route which reached clinical significance. A reduced MR (not clinically significant) was reported by Krasnow et al.[18] which could be because of more physiological serum levels of estradiol at the time of implantation. Krasnow et al Mackens et al.[25] showed that with oral route early pregnancy loss is higher due to an asynchrony between embryo and endometrium, which was also seen with our study. Ferrer-Molina et al.[26] showed that duration of estradiol treatment is reduced with transdermal route which affects live-birth rate. They also noted that patients were more comfortable with oral drug but they used transdermal patches. The patches detach and often cause local irritation. In our study, we used transdermal E2 gel which has no such problems. Bourdon et al.[27] also said that prolonged E2 exposure for endometrial preparation significantly reduces the live-birth rate and increases early pregnancy loss. The transdermal gel forms a subcutaneous estradiol depot from which drug is released slowly, maintaining a relatively stable serum level.[17] These findings match with findings of our study, where MR is higher in oral group than in the gel group.
Davar et al.[28] in 2012 compared the effects of transdermal estradiol patch and oral estradiol valerate on endometrial receptivity in FET cycles (2012). It was an randomised controlled trial (RCT) involving 90 patients with 45 in each group. There was a significant difference in estradiol level on the day of progesterone administration and the day of embryo transfer between the two groups (P = 0.001 in both), but no significant difference was observed between them in biochemical pregnancy rates and CPRs (32.6% vs. 33.3%, P = 1.000 and 30.2% vs. 33.3%, P = 0.810, respectively), which matches the findings of our study. They showed better implantation in the transdermal group but no difference in study and control groups in biochemical pregnancy rate and CPR.
Rosenwak et al[29] and Schmidt et al.[30] showed no significant difference in terms of pregnancy between the two groups which was the case with our study too. Additionally, elevated estrogen levels during IVF cycles can cause increased chances of adverse outcomes in pregnancy such as pre-eclampsia, intrauterine growth restriction (IUGR), low birth weight (LBW) babies.[31,32] One problem with transdermal route is that spontaneous ovulation rate was higher leading to cycle cancellation as was shown by Corroenne et al.[33] This can be overcome by adding GnRHa pretreatment.
Most of the studies have compared transdermal estradiol patch with oral estradiol tablets for endometrial preparation in FET cycles. Patients have complained of cumbersome use of transdermal patches due to chances of separation of patches and also local irritation. We have used transdermal estradiol gel instead of patches in our study. We have found only three studies comparing transdermal estradiol gel with oral estradiol valerate tablets for this purpose.
Xiah-hHua S et al.[34] studied a total of 244 patients who underwent HR-FET cycles in 2013 and studied endometrium and IRs after transdermal estradiol gel and vaginal progesterone versus oral estradiol and vaginal progesterone in oocyte donation program and found transdermal route as effective as oral and better tolerated, which was similar to our study. Also they found serum follicular levels were three times higher with oral estrogen in comparison to transdermal route when used for endometrial preparation in donor oocyte program. But they studied only in donor oocyte cycle and used vaginal progesterone in all the cases.
Shahrokh Tehraninejad Tehranninejad et al.[35] compared the effects of transdermal estrogen (estrogel) with oral estradiol valerate on pregnancy rates in 100 patients undergoing FET cycles in 2016 in a randomized clinical trial, after suppression with gonadotropin-releasing hormone agonist. Pregnancy rates (chemical, clinical, and ongoing), abortion rate, live-birth rate, and frequency of complications were compared between the two groups. None of the groups showed any complication. They found that biochemical pregnancy rates and CPRs were not significantly different between the two groups (P = 0.384), as reported in our study.
Garimella et al.[36] also compared transdermal gel with oral estradiol tablets for endometrial preparation FET cycles in a prospective study and included 294 HR-FET cycles in 2019. They found no significant difference in ET on the day of progesterone initiation (9.379 ± 0.96 vs. 9.465 ± 1.06, P = 0.470), the duration of E2 administration (17.19 ± 2.75 vs. 17.82 ± 3.126, P = 0.069), IR (51.9% vs. 52.1%, P = 0.792), CPR per embryo transfer (68.5% vs. 70.2%, P = 0.752), and MR (14.9% vs. 13.4%, P = 0.810) between oral and gel groups, respectively. But patient satisfaction score was significantly higher with gel (6.96 ± 0.99 vs. 8.02 ± 1.07, P < 0.01) and side effects were lesser in gel group which also corresponded with our study. But this study was not randomized.[37,38]
We have compared transdermal estradiol gel with oral estradiol tablet without GnRHa suppression in FET cycles (donor and nondonor cycles) and our study is a prospective randomized study.
Limitations
The limitation of the study were
Our study has small sample size.
Ongoing pregnancy rate and live-birth rate comparison could not be carried out as the study was of shorter duration.
CONCLUSION
This study shows no clinically significant differences in CPR, biochemical pregnancy rate, and MR between the two methods of endometrial preparation using transdermal estradiol gel and oral estradiol tablets but better endometrial thickness with transdermal route which reached clinical significance. There was reduced MR (not clinically significant) which could be because of more physiological serum levels of estradiol at the time of implantation. Our study has shown that transdermal E2 gel is comparable to oral E2 tablets in terms of endometrial thickness cycle outcomes and had lesser side effects and better patient tolerability. Hence, the transdermal gel can be preferred in patients who are intolerant to oral estrogens or patients with high BMI, patients with hypertension, diabetes mellitus (DM), with risk of venous thromboembolism (VTE), cardiovascular disease (CVD), or patients with deranged lipid profile.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ACKNOWLEDGMENT
The authors are thankful to all the participants of this study and entire infertility center staff for their valuable contribution for this study.
REFERENCES
- Comparison of early pregnancy and neonatal outcomes after frozen and fresh embryo transfer in ART cycles. J Assist Reprod Genet. 2010;27:695-700.
- [Google Scholar]
- Frozen-thawed embryo transfer: influence of clinical factors on implantation rate and risk of multiple conception. Hum Reprod. 2001;16:2316-9.
- [Google Scholar]
- Live birth rates in the first complete IVF cycle among 20 687 women using a freeze-all strategy. Hum Reprod. 2018;33:924-9.
- [Google Scholar]
- Textbook of Assisted Reproductive Techniques. In: Clinical Perspectives (4th). United States: CRC Press; 2012.
- [Google Scholar]
- International Committee for Monitoring Assisted Reproductive Technology (ICMART) world report: assisted reproductive technology2003. Fertil Steril. 2011;95:2209-22.
- [Google Scholar]
- Freeze-all policy: fresh vs. frozen-thawed embryo transfer. Fertil Steril. 2015;103:1190-3.
- [Google Scholar]
- Synchronization between embryo development and endometrium is a contributing factor for rescue ICSI outcome. Reprod Biomed Online. 2012;24:527-31.
- [Google Scholar]
- Controlled preparation of the endometrium with exogenous steroids for the transfer of frozen-thawed pre-embryos in patients with anovulatory or irregular cycles. Hum Reprod. 1991;6:443-5.
- [Google Scholar]
- Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99:156-62.
- [Google Scholar]
- Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12:731-46.
- [Google Scholar]
- Artificial endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with depot gonadotropin releasing hormone agonist in women with regular menses. J Fam Reprod Health. 2015;9:1-4.
- [Google Scholar]
- The effect of adding oral oestradiol to progesterone as luteal phase support in ART cycles − a randomized controlled study. Arch Med Sci. 2011;7:112-6.
- [Google Scholar]
- Comparison of transdermal versus oral estradiol on endometrial receptivity. Fertil Steril. 1996;65:332-6.
- [Google Scholar]
- Cryopreserved embryo transfer: endometrial preparation and timing. Semin Reprod Med. 2015;33:145-52.
- [Google Scholar]
- Preparation of cycles for cryopreservation transfers using estradiol patches and Crinone 8% vaginal gel is effective and does not need any monitoring. Eur J Obstet Gynecol Reprod Biol. 2002;103:43-7.
- [Google Scholar]
- Pharmacokinetics and pharmacodynamics of transdermal dosage forms of 17β-estradiol: comparison with conventional oral estrogens used for hormone replacement. Am J Obstet Gynecol. 1985;152:1099-106.
- [Google Scholar]
- Comparative endocrinological and clinical effects of percutaneous estradiol and oral conjugated estrogens as replacement therapy in menopausal women. Maturitas. 1991;13:297-311.
- [Google Scholar]
- Conversion of estrogen to estradiol and estradiol to estrone in postmenopausal women. Obst Gynecol. 1985;66:361-5.
- [Google Scholar]
- Frozen embryo transfer: a review on the optimal endometrial preparation and timing. Hum Reprod. 2017;32:2234-42.
- [Google Scholar]
- Oral versus transdermal oestrogen delivery for endometrial preparation before embryo transfer: a prospective, comparative, randomized clinical trial. Reprod Biomed Online. 2018;37:693-702.
- [Google Scholar]
- Prolonged estrogen (E2) treatment prior to frozen-blastocyst transfer decreases the live birth rate. Hum Reprod. 2018;33:905-13.
- [Google Scholar]
- Comparison of the effects of transdermal estradiol and estradiol valerate on endometrial receptivity in frozen-thawed embryo transfer cycles: a randomized clinical trial. J Reprod Infertil. 2016;17:97-103.
- [Google Scholar]
- Donor eggs: their application in modern reproductive technologies. Fertil Steril. 1987;47:895-909.
- [Google Scholar]
- Transfer of cryopreserved-thawed embryos: the natural cycle versus controlled preparation of the endometrium with gonadotropin-releasing hormone agonist and exogenous estradiol and progesterone (GEEP) Fertil Steril. 1989;52:609-16.
- [Google Scholar]
- High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod BioMed Online. 2010;21:331-7.
- [Google Scholar]
- Supraphysiologic estradiol is an independent predictor of low birth weight in full-term singletons born after fresh embryo transfer. Hum Reprod. 2017;32:1410-7.
- [Google Scholar]
- Endometrial preparation for frozen-thawed embryo transfer in an artificial cycle: transdermal versus vaginal estrogen. Sci Rep. 2020;10:985.
- [Google Scholar]
- Study of the endometrium and implantation rates after replacement by percutaneous estradiol and vaginal progesterone in oocyte donation programme. J Reprod Med. 2014;23:29-32.
- [Google Scholar]
- Trans dermal estrogen (oestrogel) for endometrial preparation in freeze embryo transfer cycle: an RCT. Int J Reprod Biomed (Yazd). 2018;16:51-6.
- [Google Scholar]
- A prospective study of oral oestrogen versus transdermal estrogen (gel) for hormone replacement frozen embryo transfer cycles. Gynecol Endocrinol. 2021;37:515-8.
- [Google Scholar]
- Transdermal versus oral estrogen: clinical outcomes in patients undergoing frozen-thawed single blastocyst transfer cycles without GnRHa suppression, a prospective randomized clinical trial. J Assist Reprod Genet. 2019;36:453-9.
- [Google Scholar]
- The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270-83.
- [Google Scholar]







