Translate this page into:
Progestin primed ovarian stimulation protocol: current status in assisted reproductive technology
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Progestin primed ovarian stimulation (PPOS) is a novel controlled ovarian stimulation protocol. Prevention of endogenous luteinizing hormone surge is an important step in assisted reproductive technology cycles. Gonadotropin-releasing hormone (GnRH) analogs are used conventionally for preventing premature ovulation before egg retrieval. However, GnRH analogs – both agonists and antagonists – have certain disadvantages such as increased cost, the requirement of daily injections, patient inconvenience, and adverse effects, which have prompted us to search for possible alternatives. Oral progesterone has been used recently as an effective alternative to GnRH analogs. Although the use of progestin requires mandatory cryopreservation of all the embryos due to embryo-endometrium asynchrony, advances in freezing techniques have rendered it feasible. Moreover, progestin is cost-effective, administered orally, convenient, and easily accessible. Hence, this new PPOS protocol combined with freeze-all cycles is an effective alternative to GnRH analogs for pituitary suppression during ovarian stimulation. This review aims to evaluate the current status of the PPOS protocol, its mechanism, different regimes, advantages, and disadvantages.
Keywords
Conventional progestin primed ovarian stimulation
flexible progestin primed ovarian stimulation
progestin primed ovarian stimulation
INTRODUCTION
Ovarian stimulation protocols for assisted reproductive technology (ART) cycles involve multi-follicular growth by controlled ovarian stimulation with exogenous gonadotropins, prevention of endogenous luteinizing hormone (LH) surge, and induction of final oocyte maturation by human chorionic gonadotropin or gonadotropin-releasing hormone (GnRH) analogs or both. GnRH analogs have been used conventionally for preventing premature ovulation before egg retrieval. However, GnRH analogs, both agonists and antagonists, have certain disadvantages such as increased cost, the requirement of daily injections, patient inconvenience, and some adverse effects, which have prompted us to search for possible alternatives. GnRH agonists might result in ovarian cysts, ovarian hyperstimulation syndrome (OHSS), and estrogen deficiency symptoms, whereas GnRH antagonists might be associated with an increased rate of cycle cancellation, a smaller number of oocytes retrieved, and few cases of premature LH surge in spite of using antagonist.
Oral progesterone has recently emerged as an effective alternative to GnRH analogs in preventing LH surge.[1] Progesterone secreted from the corpus luteum during the luteal phase of the natural menstrual cycle suppresses the LH surge.[2] This is the basis of replacing GnRH analogs with progesterone for preventing endogenous LH surge. The early exposure of endometrium to progesterone results in embryo-endometrium asynchrony.[3] Thus the use of progestin makes fresh embryo transfer impossible. It requires the cryopreservation of all the embryos followed by frozen embryo transfer later. Moreover, progestin is cheaper, administered orally, convenient, and easily acceptable. First developed by Kuang etal., this new progestin primed ovarian stimulation (PPOS) protocol combined with freeze-all cycles is an effective alternative to GnRH analogs for pituitary suppression during ovarian stimulation.[3] This review aims to elucidate the current status of the PPOS protocol, its mechanism, different regimes, advantages, and disadvantages.
MECHANISM OF ACTION OF PROGESTINS IN THE PREVENTION OF ENDOGENOUS LUTEINIZING HORMONE SURGE
The major regulator of gonadotropin secretion is the feedback mechanism of ovarian steroid hormones on the anterior pituitary.[2] In the early follicular phase, estrogen secreted from the granulosa cells of the follicles exerts negative feedback on the secretion of gonadotropins from the pituitary. On prolonged estrogen exposure, when it reaches a critical threshold, the oestradiol exerts positive feedback resulting in the pulsatile release of GnRH and hence the LH surge and ovulation [Figure 1].

- Conventional Ovarian Stimulation Protocols
Progesterone is also an important hormone in the ovulatory cascade. It acts through progesterone (P) receptors and blocks the estrogen mediated pulsatile release of GnRH and LH surge.[4,5] The exact mechanism behind this blockade is not clear. Estrogen upregulates progesterone receptors but progesterone downregulates its own receptors. The action of progesterone depends on its concentration and time of exposure. The mildly elevated levels of progesterone in the late follicular phase stimulate the LH surge for a narrow time frame, whereas higher levels of progesterone block the LH surge and ovulation as seen in the luteal phase, with contraceptives or with pregnancy.[4,5,6] The continuous high progesterone concentration above its physiological trigger level desensitizes its own and GnRH receptors, thereby inhibiting LH surge and thus blocking ovulation. Thus, this inhibitory effect of the continuous high level of progesterone on gonadotropin secretion is the pillar of PPOS.
INDICATIONS
PPOS may be preferred over GnRH analogs for preventing premature LH surge in cycles planned for frozen embryo transfer. This includes the following:
Oocyte donors
Fertility preservation
Nonconventional protocols such as luteal phase stimulation and dual stimulation
Preimplantation genetic testing for aneuploidy and monogenic diseases
Patients at risk of OHSS like polycystic ovarian syndrome
Patients with poor ovarian reserve
PROGESTIN PRIMED OVARIAN STIMULATION PROTOCOL
Conventional PPOS protocol: Medroxyprogesterone acetate (MPA) 10 mg/day is administered from D2 or D3 of the cycle simultaneously with gonadotropin till the day of ovulation trigger.[7,8]
Flexible PPOS protocol (fPPOS): MPA 10 mg/day is started from day 7 or when the leading follicle reaches 14 mm, whichever is first, and continued till the day of trigger. Gonadotropin is started fromday 2 of the cycle till the day of trigger [Figures 2 and 3].[9]
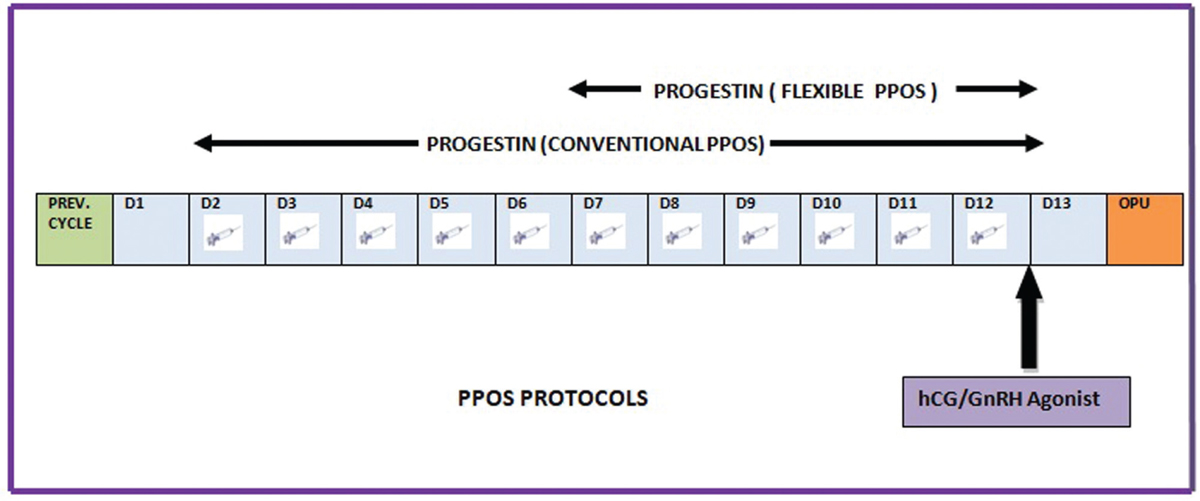
- Progestin Primed Ovarian Stimulation Protocol

- Mechanism of Progestin Primed Ovarian Stimulation
PROGESTIN TYPE AND DOSE
MPA: It is the most commonly used progestin. It is given in a dose of 10 mg/day either from day 2 or 7 of the cycle. Dong etal. compared a 4 mg/day dose of MPA with a 10 mg/day dose and it was found to be equally effective.[10]
Dydrogesterone: It is given in a dose of 20 mg/day starting from day 3 of cycle till the day of trigger. It is found to be equally effective as compared to MPA.[11]
Micronized progesterone: Utrogestan Abbott Healthcare, Abbott USA can be given in a dose of 100 or 200 mg/day from day 3 along with hMG till the day of trigger. It is equally effective.[12]
EFFICACY OF PROGESTINS IN PROGESTIN PRIMED OVARIAN STIMULATION
Progestins have been shown in many studies to prevent premature ovulation as efficiently as GnRH analogs.[12,13,14] Since 2015, various studies have been done comparing this novel PPOS with GnRH agonist or GnRH antagonist, the efficacy of different progestins, and varied dose and duration of the same progestin.[13,14,15,16] The main outcomes compared between the two groups were duration of stimulation, gonadotropin consumption, the total number of mature oocytes retrieved, the total number of embryos, incidence of premature LH surge, implantation rate, clinical pregnancy rate (CPR), and live birth rate (LBR). The important studies with the major parameters are summarized in Table 1.

A) PPOS versus GnRH analogs
Kuang etal. in 2015 compared MPA with GnRH agonist for prevention of LH surge in a controlled ovarian stimulation cycle. In the study group, MPA 10 mg/day was administered along with human menopausal gonadotrophin (HMG) from day 3 of the cycle till the day of trigger. In the control group, as per the short protocol triptorelin (0.1 mg/day) from day 2 cycle was given along with HMG from day 3 of the menstrual cycle. Premature LH surge occurred in only one case in the study (MPA) group as compared to progestin. Higher doses of HMG were administered in the MPA group. There was no significant difference between the number of oocytes retrieved, number of embryos, the incidence of premature LH surge, CPR, and LBR.[3]
Chen etal., in 2019, compared MPA 10 mg/day with GnRH antagonist in the prevention of premature LH surge in poor responders. The incidence of spontaneous LH surge and premature ovulation was significantly lower in the MPA group as compared to the GnRH antagonist cycle (0 vs. 5.88%, P < 0.05).[8]
Beguería etal. compared MPA with GnRH antagonists in oocyte donation cycles. MPA was started simultaneously with gonadotropins like in other previous studies. There was no significant difference in terms of duration of stimulation, total gonadotropin consumption, and the number of mature oocytes retrieved. The CPR was significantly lower in the MPA group (31%) as compared to the GnRH antagonist group (46%) (P = 0.006). But there was no significant difference between the LBR (22% in the MPA group and 31% in the antagonist group) (P = 0.10).[13]
Yildiz etal. in 2019 evaluated a different fPPOS in oocyte donors. Within 6 months, each oocyte donor was administered a flexible GnRH antagonist in one cycle and the novel fPPOS protocol in the other. All patients were stimulated with gonadotropins from cycle day 2 or 3, along with the addition of 0.25 mg/day GnRH antagonist or 10 mg/day MPA from stimulation day 7 or when the leading follicle was 14 mm, whichever came first. Total gonadotropin consumption and duration of stimulation were similar. There was no premature ovulation in any group. The fPPOS protocol resulted in a significantly higher number of oocytes retrieved (P = 0.02) but similar live birth.[9]
Giles etal. in 2021 compared the efficacy of MPA versus GnRH antagonists for pituitary suppression in oocyte donors undergoing ovarian stimulation. There was no significant difference in oocyte retrieval rate, the total number of embryos, and the pregnancy rate among both the groups.[14]
B) Different progestins for PPOS
Progestins other than MPA have also been evaluated in PPOS protocols. Zhu etal. conducted a retrospective study comparing oral utrogestan (200 mg/day) along with gonadotropin versus a short protocol. There was no significant difference in the number of mature oocytes or ongoing pregnancy rate.[15]
Yu etal. have explored dydrogesterone 20 mg/day versus MPA 10 mg/day in the PPOS stimulation cycle. This prospective randomized study has shown similar rates of oocyte retrieval and pregnancy outcome.[11]
C) Different doses of the same progestin
Dong etal. conducted a randomized trial to find out the minimum dose of MPA in PPOS comparing 4 mg MPA with 10 mg of MPA. The difference in both the groups in major outcomes compared was statistically insignificant. MPA at a dose of 4 mg was comparable to a 10 mg dose in terms of efficacy.[10]
Zhu etal. conducted a prospective controlled study comparing utrogestan 200 mg versus 100 mg/day as a progestin in PPOS protocol. There was no significant difference in the number of oocytes, mature oocytes, viable embryos, or CPR.[12]
MERITS
PPOS is a promising approach for LH surge prevention in ovarian stimulation cycles without impairing the oocyte retrieval rate, quality of embryos, pregnancy, and LBR.
Progestins can be used orally as compared to GnRH analog injections.
They are cheaper and thus cost-effective.
Total cryopreservation of embryos followed by frozen embryo transfer is mandatory. Thus, it avoids the potential adverse effect of increased hormonal levels on endometrial receptivity.
Progesterone priming is a promising approach to prevent premature ovulation in the case of oocyte donors, fertility preservation, preimplantation genetic testing cycle, nonconventional ovarian stimulation protocols like luteal phase and random start and dual stimulation, and patients at risk of OHSS planned for frozen cycles.
Freeze-all cycles with delayed transfer can decrease the risk of late-onset OHSS.
Other advantages include its easy accessibility, being patient-friendly, and greater control over LH concentrations.[1]
DEMERITS
Progestins precludes fresh embryo transfer as early raised levels of progesterone have a negative effect on the endometrium. It can be applied only to planned freeze-all cycles.
Total cryopreservation and delayed transfer increase the time of pregnancy.
Some studies have shown that raised progesterone level on the day of trigger significantly reduces the rate of top-quality blastocyst formation.[16] However, the quality of assessment is quite subjective.
Few studies have shown increased gonadotropin dose required in PPOS cycles and increased cost due to additional monitoring and thawing of embryos for embryo transfer and thereby an overall increase in cost.[17] Thus, PPOS is cost-effective only in the case of planned freeze-all cycles.
CONCLUSION
PPOS is a promising patient-friendly protocol for controlled ovarian stimulation. It can prove revolutionary as it replaces the GnRH antagonist or agonist injections with oral pills. Thus, it has increased acceptance and it is convenient and cost-effective. But it can be used only in a planned freeze-all cycles. However, more prospective studies are needed with a large study population to completely replace GnRH analogs with oral progestins for the prevention of premature LH surge in ART cycles.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Controlled Ovarian Stimulation Using Medroxyprogesterone Acetate and hMG in Patients With Polycystic Ovary Syndrome Treated for IVF: A Double-Blind Randomized Crossover Clinical Trial. Medicine. 2016;95:e2939. doi: https://doi.org/10.1097/MD.0000000000002939
- [Google Scholar]
- Ovarian feedback, mechanism of action and possible clinical implications. Hum Reprod Update. 2006;12:557-71.
- [Google Scholar]
- Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril. 2015;104:62-70.e3. doi: https://doi.org/10.1016/j.fertnstert.2015.03.022
- [Google Scholar]
- New stimulation regimens: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update. 2017;23:211-20.
- [Google Scholar]
- On the site of action of progesterone in the blockade of the estradiol-induced gonadotropin discharge in the rhesus monkey. Endocrinology. 1981;109:1293-4.
- [Google Scholar]
- Progesterone can block the preovulatory gonadotropin-releasing hormone/luteinising hormone surge in the ewe by a direct inhibitory action on oestradiol-responsive cells within the hypothalamus. J Neuroendocrinol. 2005;17:161-9.
- [Google Scholar]
- Progesterone is a physiological trigger of ovulatory gonadotropins. Fertil Steril. 2020;113:923-4.
- [Google Scholar]
- Progestin vs. gonadotropin-releasing hormone antagonist for the prevention of premature luteinizing hormone surges in poor responders undergoing in vitro fertilization treatment: a randomized controlled trial. Front Endocrinol. 2019;10:796. doi: https://doi.org/10.3389/fendo.2019.00796
- [Google Scholar]
- Comparison of a novel flexible progestin primed ovarian stimulation protocol and the flexible gonadotropin-releasing hormone antagonist protocol for assisted reproductive technology. Fertil Steril. 2019;112:677-83.
- [Google Scholar]
- The pregnancy outcome of progestin-primed ovarian stimulation using 4 versus 10 mg of medroxyprogesterone acetate per day in infertile women undergoing in vitro fertilisation: a randomised controlled trial. BJOG. 2017;124:1048-55.
- [Google Scholar]
- New application of dydrogesterone as a part of a progestin-primed ovarian stimulation protocol for IVF: a randomized controlled trial including 516 first IVF/ICSI cycles. Hum Reprod. 2018;33:229-37.
- [Google Scholar]
- Use of Utrogestan during controlled ovarian hyperstimulation in normally ovulating women undergoing in vitro fertilization or intracytoplasmic sperm injection treatments in combination with a “freeze all” strategy: a randomized controlled dose-finding study of 100 mg versus 200 mg. Fertil Steril. 2017;107:379-386.e4. doi:10.1016/j.fertnstert.2016.10.030
- [Google Scholar]
- Medroxyprogesterone acetate versus ganirelix in oocyte donation: a randomized controlled trial. Hum Reprod. 2019;34:872-80.
- [Google Scholar]
- Medroxyprogesterone acetate is a useful alternative to a gonadotropin-releasing hormone antagonist in oocyte donation: a randomized, controlled trial. Fertil Steril. 2021;116:404-12.
- [Google Scholar]
- Utrogestan as an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Medicine (Baltimore). 2015;94:e909. doi:10.1097/MD.0000000000000909
- [Google Scholar]
- Use of progestins to inhibit spontaneous ovulation during ovarian stimulation: the beginning of a new era? Reprod Biomed Online. 2019;39:321-31.
- [Google Scholar]
- Progestins for pituitary suppression during ovarian stimulation for ART: a comprehensive and systematic review including meta-analyses. Hum Reprod Update. 2021;27:48-66.
- [Google Scholar]







