Translate this page into:
Does dual trigger with gonadotropin-releasing hormone agonist and low-dose human chorionic gonadotropin improves clinical outcomes in normal responders in gonadotropin-releasing hormone (GnRH) antagonist IVF-ICSI Cycles
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
To study and compare the effectiveness of dual trigger with gonadotropin-releasing hormone agonist (GnRHa) and low dose human chorionic gonadotropin (hCG) for final oocyte maturation versus human chorionic gonadotropin trigger for improving clinical outcomes in GnRH Antagonist IVF-ICSI Cycles in normal responder patients.
Materials and Methods:
A prospective comparative study was conducted at a tertiary care infertility centre. Eighty (80) normoresponders who were to undergo IVF-ICSI cycles were included in the study. Patients in both groups underwent controlled ovarian hyperstimulation using GnRH antagonist protocoI. Group 1/Study (n = 40) patients were given Inj Leuprolide acetate and low dose highly purified injection hCG. Group 2/ control group received standard dose of highly purified injection hCG. Day 3 fresh embryo transfers were performed. Primary outcome measured was clinical pregnancy rate and secondary outcomes measured were: implantation rate, miscarriage rate, number of MII oocytes, number of embryos formed, risk of OHSS.
Results:
In group 1 the clinical pregnancy rate and implantation rate were higher than in group 2, 52.50% vs 47.50% and 29.67% vs 26.08% but the difference was not statistically significant. There were significantly higher number of MII oocytes retrieved (10.63±5.46 Vz 8.10±5.74) and higher number of embryos formed (8.2±3.4 Vz 6.8±3.6) in group1 than in group 2.
Conclusion:
Though there was increased clinical pregnancy rate and implantation rate in dual trigger group but it was not statistically significant. There was significant increase in MII oocytes and number of embryos formed in the dual trigger group.
Keywords
Dual trigger
Dual trigger in GnRH antagonist IVF cycle
Ovarian stimulation in IVF
INTRODUCTION
Twenty years ago, Gonel et al. in 1990 introduced gonadotropin-releasing hormone (GnRH) agonist for triggering the final oocyte maturation in place of human chorionic gonadotropin (hCG).[1] GnRH agonist as trigger in GnRH antagonists protocols for controlled ovarian stimulation (COH) became popular due to advantage of drastic reduction in ovarian hyperstimulation syndrome (OHSS).[2,3,4]
GnRH antagonist cotreatment during ovarian stimulation allows ovulation to be induced with a bolus of GnRHa, as GnRHa displaces the GnRH antagonist in the pituitary, activating the GnRH receptor, resulting in a surge of gonadotrophins (flare-up), similar to that of the natural midcycle surge of gonadotrophins as it causes endogenous release of both follicle-stimulating hormone (FSH) and luteinizing hormone (LH).[5] Mean duration of LH surge is shorter about 24 hours, it is very similar to the natural cycle duration of 48 hours,[6] and thus reducing the incidence of OHSS in high responders.[7] However, there have been some issues with the substitution of GnRH-agonists as trigger.
Kummer et al. in 2013 discovered that the risk of empty follicle syndrome was increased following isolated GnRH-agonist trigger due to a suboptimal LH surge in a subset of patients.[8] Also there have been increased early pregnancy loss and decreased rates of ongoing pregnancy as noted by multiple studies.[9]
Hoff et al. in 1983 postulated that significant differences are there between the GnRHa-induced surge and that of the natural cycle LH-surge. There are three phases in LH-surge of natural cycle,[6] with a total duration of 48 hours, on the other hand the GnRHa-induced surge of gonadotrophins consists of only two phases, with duration of 24 to 36 hours as described by Itskovitz et al. in 1991.[9] This results in a significantly reduced total amount of gonadotrophins being released from the pituitary when GnRHa is used to trigger final oocyte maturation.[1,9] Hence, the combined effect of ovarian stimulation and GnRHa trigger decreases the endogenous LH concentration quite dramatically during the early luteal phase,[10] requiring a proper adjustment of the standard luteal-phase support to safeguard the reproductive outcome.[10]
However, with hCG there is an advantage of luteal-phase support due to its long duration of action because the hCG-mediated LH activity spans the luteal phase for several days, unlike the physiological midcycle surge of LH and FSH, which terminates 48 hours after its onset.[11] Multiple corpus luteum get stimulated by this supra-physiological LH activity, resulting in high-serum progesterone and oestradiol concentrations, which in turn reduce the endogenous LH secretion by the pituitary.[11] Humaidan et al. in 2012 explained that hCG given for final oocyte maturation covers the luteal phase for a total of 8 to 10 days and all luteal actions of LH gets covered including the upregulation of vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF2) and cytokines (LIF) necessary for successful implantation to take place.[12] Later this function is covered by the hCG produced by the implanting embryo.[12] Hence, dual trigger can give the benefits of a bolus of a GnRHa in terms of release of endogenous LH and FSH from the pituitary combined with the long-acting LH activity of a small bolus of hCG, covering the early luteal-phase LH deficiency. The dual trigger protocol is usually followed by a standard luteal-phase support.
“Dual trigger” was first defined in 2008as the concept of a combination of GnRH-a and a low-dose hCG in triggering final oocyte maturation by Shapiro et al. in a retrospective study in patients treated with GnRH antagonist cycle for the purpose of preventing OHSS[13] in high ovarian responders and showed improved implantation rate, clinical pregnancy, and live birth rates in normal responders using dual trigger regimen.
Since then, many studies have shown benefits of using dual triggering regimen in high and poor responders patients but few studies have been done to show their usefulness for normal responder patients.[14,15]
The aim of this study was to study and compare the effectiveness of dual trigger with gonadotropin-releasing hormone agonist (GnRHa) and low-dose hCG for final oocyte maturation with hCG trigger for improving clinical outcomes in GnRH Antagonist IVF-ICSI Cycles in normal responder patients.
MATERIALS AND METHODS
STUDY DESIGN
A single-center, prospective randomized comparative study was conducted at a tertiary care infertility center from 1st March 2020 to 28th February 2021.
Total 118 patients who were to undergo controlled ovarian hyperstimulation for IVF-ICSI cycles during the study period at the center were assessed for eligibility, for which patients underwent clinical history taking, physical examination, complete hematological and biochemical screening to look for inclusion and exclusion criteria.
Eighty (80) patients who were normal responders were included in the study according to inclusion and exclusion criteria. Normal responders were defined as women in age group 21 to 37 years, with serum AMH level >1.2 ng/mL to <4 ng/mL, with AFC (3–8) per ovary
Inclusion criterion were women: (i) aged 21 to 37 years; (ii) with a body mass index (BMI) of ≥18–≤25 kg/m2; (iii) who had a normal response to controlled ovarian stimulation (6–20 retrieved oocytes); and (iv) a normal uterine cavity assessed by 2D ultrasonography (USG)/hysteroscopy.
Exclusion criteria were women: (i) with a hyper response (number of retrieved oocytes >20) or weak response (number of retrieved oocytes <6) to controlled ovarian hyperstimulation (COH); (ii) with >3 attempts at IVF and/or ICSI; (iii) suffering from an endocrine disorder (diabetes mellitus, hyperprolactinemia, thyroid dysfunction, congenital adrenal hyperplasia, Cushing syndrome, or polycystic ovary syndrome), or a uterine anomaly confirmed by hysterosalpingography or hysteroscopy; and (iv) untreated hydrosalpinx.
Sample size calculation: All consecutive patients meeting the eligibility criteria during the study period were enrolled. With reference to previous studies and their experience, it was decided that atleast 40 cases per group should be enrolled (total = 80) for a meaningful observation.
Embryo scoring/grading: all embryos were graded according to Istanbul Consensus, 2011 (Human Reprod, Vol 0(0).1–14, 2011) and only Day 3 embryos of grade 1 or grade 2 quality were transferred in all cases.
Methodology: After ethical clearance and proper informed consent, patients were enrolled into one of the two groups randomly by a computer-generated randomization program.
Group 1/study group (n = 40) patients were given Inj Leuprolide acetate (Sun Pharma, India) and low-dose highly purified injection hCG (Sun Pharma, India) subcutaneously. Group 2/control group (n = 40) received standard dose of highly purified injection hCG (Sun Pharma, India). All patients underwent ovarian stimulation with a flexible starting dose of recombinant FSH (Intas, India) with dosage 150–225 IU on day 2 of menses and was continued for 4 days at the same dosage then the dosage was adjusted according to follicular monitoring with serial transvaginal (TVS) two dimensional (2D) ultrasonography. After at least one follicle reached a size of 14 mm, antagonist injection (inj cetrorelix 0.25 mg, Zydus, India) started subcutaneously daily. When > 2 follicles reached the size of 18 mm, final oocyte maturation was triggered by either a combination of Inj Leuprolide acetate 2 mg and low-dose highly purified hCG inj 2000 IU (dual trigger) in Group 1 or by a standard dose of highly purified hCG inj 10,000 IU subcutaneouly in Group 2. Oocyte retrievals were performed under transvaginal ultrasound guidance 35 to 36 hours after the trigger. IVF-ICSI was performed in all cases. Day 3 fresh embryo transfers were performed for all cases (2 × 8 cell embryos) 72 hours after oocyte retrieval on day 4 of progesterone start. Endometrial thickness was checked using 2D-TVS before transferring the embryos. The catheters (soft catheter) used for embryo transfer were the same. All transfers were done by a single clinician to avoid bias from difference in skills of the clinicians. All ultrasonographic examinations were performed by one clinician.
The luteal-phase support was with vaginal supplementation of 800 mg micronized progesterone starting on the day of oocyte retrieval. Urine pregnancy test was performed after 14 days of embryo transfer, if positive it was followed by serum beta-hCG test. A value of more than 25 U/mL in serum beta-hCG test was considered to be a positive pregnancy. The luteal support was given until 10th week of pregnancy.
Primary outcome measured was clinical pregnancy rate and secondary outcomes measured were: implantation rate, miscarriage rate, number of MII oocytes, number of embryos formed, risk of OHSS.
Biochemical pregnancy rate was defined as a serum beta-hCG level greater than 25 IU/L 14 days after embryo transfer. Clinical viable pregnancy (CPR) was taken as the presence of a gestational sac with heart beat identified by vaginal/abdominal USG at 7 weeks period of gestation. Implantation rate (IR) was determined as the ratio of gestational sacs to the number of embryos transferred and miscarriage was regarded as pregnancy loss before 12 weeks of gestation.
ETHICAL CONSIDERATION: The study was approved by the Ethics Committee Review Board of Indian Fertility Society and written informed consent was obtained from all participants after giving them all the needed information.
STASTICAL ANALYSIS: Statistical testing was conducted with the statistical package for the social science system version IBM SPSS 19.0. For all statistical tests, a P value less than 0.05 was taken to indicate a significant difference.
RESULTS
Figure 1 shows the consort flow chart, which shows total 80 patients were recruited for this study, from among normal responder patients undergoing IVF-ICSI cycle (n = 118) during the study period and were randomized into one of the two groups according to inclusion and exclusion criteria. Group 1 (n = 40) received dual trigger (dual-trigger group) and group 2 (n = 40) received standard hCG trigger (hCG trigger group) for final oocyte maturation after COH. In both the groups, there were no cancellations; all the patients were followed up and their data analyzed for outcomes.

- Consort flow chart.
[Table 1] Table 1 shows the baseline characteristics and demographics in study and control group. Both groups were similar in age, BMI, duration and cause of infertility, antral follicle count, serum AMH level, basal FSH and E2 level.
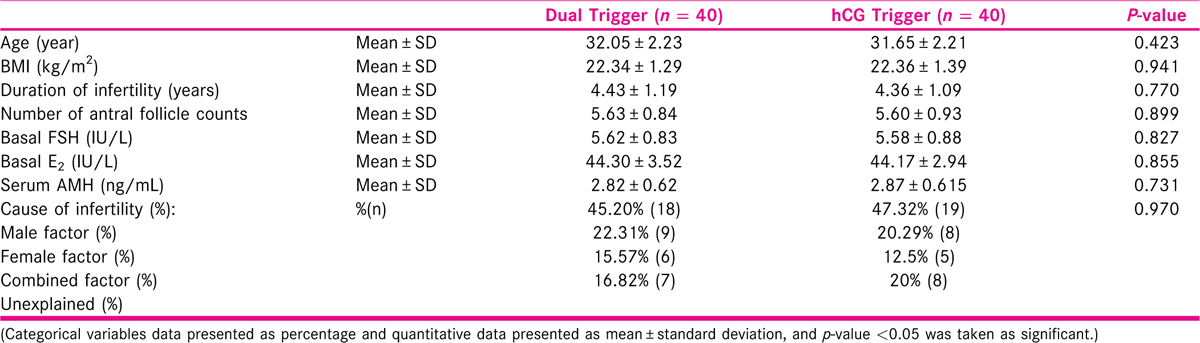
[Table 2] Table 2 shows the ovarian stimulation cycle characteristics. Both groups were comparable in stimulation cycle characteristics and the association was not found to be statistically significant.

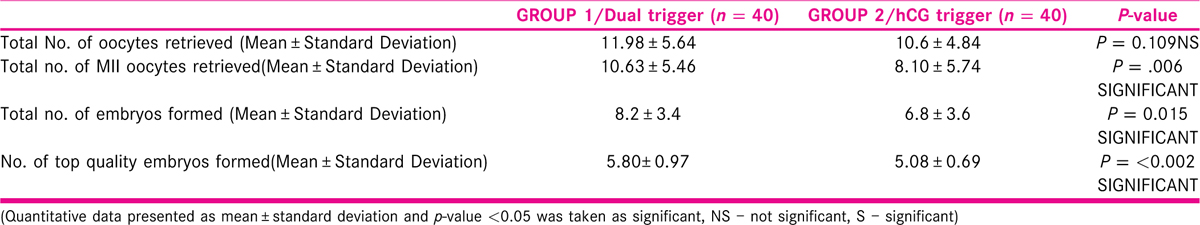
[Table 3] Table 3 shows the number, quality of oocytes retrieved, and embryos formed in the two groups. Although the total number of oocytes were similar in both the groups, the number of retrieved mature metaphase II (MII) oocytes, total number of embryos, and total number of top-quality embryos all were statistically significantly higher in the dual-trigger group.
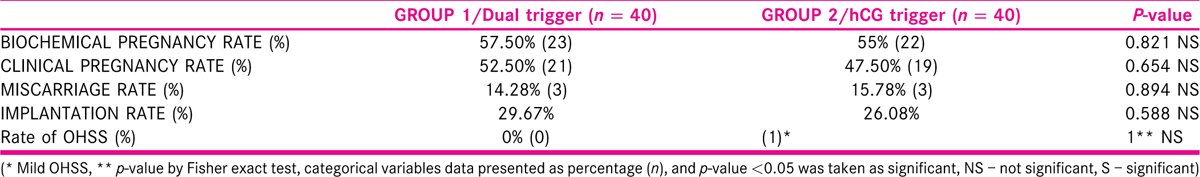
[Table 4] Table 4 shows the comparison of clinical outcomes in both the groups. In dual-trigger group (group 1), the biochemical pregnancy rate, clinical pregnancy rate, and implantation rate were higher than in hCG trigger group (group 2), 57.50% vs. 55%; 52.50% vs. 47.50%; and 29.67% vs. 26.08%, but the difference was not statistically significant (p = 0.821; p = 0.654; p = 0.588, respectively). Miscarriage rate was lesser in group 1 (14.28%) than in group 2 (15.78%), but this was also statistically not significant (p = 0.894). There were higher number of MII oocytes retrieved and higher number of embryos formed in group 1 than in group 2 and this difference was statistically significant. In group 1, there were no cases of OHSS and only one case of mild OHSS was reported in group 2, which was managed on out-patient basis.
DISCUSSION
The present study demonstrated similar implantation, clinical pregnancy, rate and total number of oocytes retrieved among patients who underwent dual triggering of oocyte maturation and those who had hCG-only triggering. Although there were higher implantation, clinical pregnancy rate in the dual-trigger group but the difference was not statistically significant. However, total number of mature MII oocytes retrieved and high-quality embryos was higher in the dual-trigger group. These data suggest that the dual trigger could improve the quantity of embryos and quality of embryos and oocytes.
As described earlier compared with hCG alone, administration of GnRH agonist induces an increase in endogenous LH and FSH release, which is very similar to the natural midcycle surge of gonadotropins.[16] This surge in FSH could activate resumption of the oocyte meiotic process and cumulus expansion at the final stage of oocyte maturation.[16] Also Lamb et al. found that administration of FSH at the time of the hCG trigger improved the rate of oocyte retrieval and 2 PN embryos significantly, and led to a greater concentration of FSH in follicular fluid as compared with using an hCG trigger alone.[17] In a study by Griffin et al., the researchers found that dual trigger increased the maturation rate of oocytes by up to 75% among women who earlier had low maturation rates during hCG trigger cycles.[18] In our study also we got significantly higher number of mature oocytes in dual-trigger group.
Although how midcycle FSH surge helps is not completely understood, but it is suggested that it enhances LH receptor formation in luteinizing granulosa cells, nuclear maturation, and cumulus expansion,[19] and thus improving oocyte maturity and pregnancy outcome. Our study was also based on this hypothesis. Our results resemble that of Lin et al. and Schachter et al. who reported that the use of dual trigger in normal responders is associated with significant increase in numbers of MII oocytes.[20,21] Also Schachter et al. showed significantly higher implantation rates among women who were given dual trigger (0.2 mg of triptorelin in combination with 5000 IU of hCG) than those who were given 5000 IU of hCG alone for trigger.[21] Whereas, Alleyassin et al. could not find any significant difference in the number of MII oocytes, the number of COCs, and the number of 2 PN oocytes clinical pregnancy rate and live birth rate with the use of dual trigger in comparison to the standard dose of hCG alone.[22]
Decleer et al. suggested that the use of dual trigger using GnRH agonist and the standard dose of urinary hCG decrease the pregnancy rate, although dual trigger group had higher number of top-quality embryos.[23] They suggested this could be explained by the negative effect on the endometrial receptivity induced by the higher LH levels and the additional FSH surge, but they used standard dose of hCG (5000 IU) with GnRH agonist (Triptorelin 0.2 mg).[23]
A previous study reported that a decrease in endometrial receptivity, rather than poor embryo quality, leads to inferior outcomes in GnRH antagonist cycles.[24] The GnRH agonist can remove the negative effects of the GnRH antagonist on endometrial receptivity and thus promoting embryo implantation, as concluded in a review of the role of GnRH receptors in mediating endometrial receptivity and embryo implantation.[25]
Kim et al. suggested that the dual-trigger group showed statistically significant improved rates of implantation, clinical pregnancy, and live birth. They explained that this could be due to multiple roles of GnRH in the regulation of endometrial receptivity and embryo implantation.[26] Although we had higher implantation rate in our study, but we could not include live birth rate in our study due to shorter duration of our study.
Zhou et al. compared the outcomes in 220 patients with dual trigger and 110 control patients and found no significant difference between the two groups regarding implantation rate, clinical pregnancy, or live births in a retrospective cohort study.[27] Whereas, Haas et al. in 2020 studied (RCT) 150 patients over a period of 2 years and found that not only the number of total oocytes, MII oocytes, top-quality embryos but also the clinical pregnancy rate and live birth were significantly more in dual-trigger group.[28]
A recent systematic review and meta-analysis conducted by Ding et al. in 2017 on dual trigger for final oocyte maturation in vitro fertilization[29] included four RCTs with different sample sizes. The final conclusion was that dual trigger was similar to hCG in triggering oocyte maturation and may be effective in improving reproductive outcomes.
Our study was prospective randomized study which is the greatest strength of our study. Also it was done in normal responders on whom only few studies have been done in the past.
The main limitation of this study is its small sample size because of which clinically significant difference in clinical outcomes could not be seen probably. Also, we could not compare ongoing pregnancy rate and live birth rate between the groups.
CONCLUSION
In conclusion, in terms of the number of MII oocytes retrieved, top-quality embryos in normal responders, a dual-trigger approach seems superior to an hCG trigger alone. Future randomized controlled trials in large cohorts and meta-analyses are needed to clarify the exact impact of dual trigger on clinical outcomes in normal responders undergoing ART cycles.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Commentary
First IVF baby was born in the year 1978. Over the last 44 years, there have been constant attempts to make IVF simpler, improve the success rates, and also to reduce the treatment burden.
In past, studies had been concentrating on getting ovarian stimulation right, but last decade had seen interest in optimising luteal phase. There have been attempts to reduce the complications such as ovarian hyperstimulation syndrome (OHSS). While GnRH agonist trigger was started to prevent OHSS, very quickly it was realised that this alone was not sufficient, and we need to add something to maintain the success rates. Addition of low dose Human chorionic gonadotropin (HCG) on day of egg collection was then started.
As every step in long process of IVF is important and any outcome will be as good as the weakest link. Recently attention has been given to the trigger injections to improve the number of oocytes. This study joins a number of recently published studies which compare the outcomes after giving trigger injection with HCG alone versus HCG and GnRHagonists (dual trigger). Theoretical basis is the improvement in endogenous Follicle stimulating hormone (FSH) and LH by GnRHagonists with adds to activity of HCG.
Although this is a very small trial with no appropriate sample size calculation, it suggests that there is no difference in live birth outcomes in the two groups. It is hard to draw definite conclusions as numbers are too small and there is no cost-effectiveness analysis.
Following pandemic and in current economic climate, we are all required to deliver more with less rather than same with more. Hence, we should be looking at reducing rather than increasing the number of injections. So, this study provides some reassurances that one injection is as good. Although conclusion is misleading in quoting that number of Metaphase II (MII) and top-quality embryo is increased as study is not powered to assess this.
What we need is an agreed dose of HCG and GnRHagonists with appropriate sample size, economic evaluation alongside clinical evaluation to be able to reach at definite conclusions. At the same time this study provides a pilot to build a definitive trial.
Dr. Abha Maheshwari, Consultant reproductive medicine, Honorary Professor, Aberdeen Maternity Hospital, Aberdeen, UK.
REFERENCES
- Use of gonadotropin-releasing hormone agonist to trigger follicular maturation for in vitro fertilization. J Clin Endocrinol Metab. 1990;71:918-22.
- [Google Scholar]
- The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomized controlled study. Fertil Steril. 2008;89:84-91.
- [Google Scholar]
- Can we eliminate severe ovarian hyperstimulation syndrome? Hum Reprod. 2005;20:320-2.
- [Google Scholar]
- Gonadotrophin-releasing hormone antagonists for assisted conception: a Cochrane review. Reprod Biomed Online. 2007;14:640-9.
- [Google Scholar]
- Effects of recombinant LH supplementation in women undergoing assisted reproduction with GnRH agonist down-regulation and stimulation with recombinant FSH: an opening study. Reprod Biomed Online. 2004;8:635-43. Epub2004/06/01
- [Google Scholar]
- Quigley ME, Yen SS. Hormonal dynamics at midcycle: a reevaluation. J Clin Endocrinol Metab. 1983;57:792-6.
- [Google Scholar]
- Elective cryopreservation of all pronuclear oocytes after GnRH agonist triggering of final oocyte maturation in patients at risk of developing OHSS: a prospective, observational proof-of-concept study. Hum Reprod. 2007;22:1348-52.
- [Google Scholar]
- Predicting successful induction of oocyte maturation after gonadotropin-releasing hormone agonist (GnRHa) trigger. Hum Reprod. 2013;28:152-9.
- [Google Scholar]
- Induction of preovulatory luteinizing hormone surge and prevention of ovarian hyperstimulation syndrome by gonadotropin-releasing hormone agonist. FertilSteril. 1991;56:213-20.
- [Google Scholar]
- Luteal phase rescue in high-risk OHSS patients by GnRHa triggering in combination with low-dose HCG: a pilot study. Reprod Biomed Online. 2009;18:630-4.
- [Google Scholar]
- Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Endocrinol Metab. 2003;4:236-42.
- [Google Scholar]
- The luteal phase after GnRH-agonist triggering of ovulation: present and future perspectives. Reprod Biomed Online. 2012;24:134-41.
- [Google Scholar]
- Gonadotropin-releasing hormone agonist combined with a reduced dose of human chorionic gonadotropin for final oocyte maturation in fresh autologous cycles of in vitro fertilization. Fertil Steril. 2008;90:231-3.
- [Google Scholar]
- Dual trigger with gonadotropin-releasing hormone and human chorionic gonadotropin for poor responders. J Turk Ger Gynecol Assoc. 2018;19:98-103.
- [Google Scholar]
- Dual trigger of oocyte maturation with gonadotropin releasing hormone agonist and low-dose human chorionic gonadotropin to optimize live birth rates in high responders. Fertil Steril. 2012;97:1316-20.
- [CrossRef] [Google Scholar]
- LH (as HCG) and FSH surges for final oocyte maturation: sometimes it takes two to tango? Reprod Biomed Online. 2010;21:590-2.
- [Google Scholar]
- Follicle-stimulating hormone administered at the time of human chorionic gonadotropin trigger improves oocyte developmental competence in in vitro fertilization cycles: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2011;95:1655-60.
- [Google Scholar]
- Dual trigger with gonadotropin-releasing hormone agonist and standard dose human chorionic gonadotropin to improve oocyte maturity rates. Fertil Steril. 2014;102:405-9.
- [Google Scholar]
- Empty follicle syndrome: successful treatment in a recurrent case and review of the literature. Hum Reprod. 2012;27:1357-67.
- [Google Scholar]
- Dual trigger with combination of gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves the live-birth rate for normal responders in GnRH-antagonist cycles. Fertil Steril. 2013;100:1296-302.
- [Google Scholar]
- Can pregnancy rate be improved in gonadotropin-releasing hormone (GnRH) antagonist cycles by administering GnRH agonist before oocyte retrieval? A prospective, randomized study. Fertil Steril. 2008;90:1087-93.
- [Google Scholar]
- Final oocyte maturation with a dual trigger compared to human chorionic gonadotropin trigger in antagonist co-treated cycles: a randomized clinical trial. Middle East Fertil Soc J. 2018;23:199-204.
- [Google Scholar]
- Comparison of hCG triggering versus HCG in combination with a GnRH agonist: a prospective randomized controlled trial. Facts Views Vis Obgyn. 2014;6:2039.
- [Google Scholar]
- Effects of GnRH antagonist on endometrial protein profiles in the window of implantation. Proteomics. 2014;14:2350-9.
- [Google Scholar]
- The role of peripheral gonadotropin-releasing hormone receptors in female reproduction. Fertil Steril. 2011;95:465-73.
- [Google Scholar]
- Combined administration of gonadotropin-releasing hormone agonist with human chorionic gonadotropin for final oocyte maturation in GnRH antagonist cycles for in vitro fertilization. J Reprod Med. 2014;59:63-8.
- [Google Scholar]
- Comparison of dual trigger with combination GnRH agonist and hCG versus hCG alone trigger of oocyte maturation for normal ovarian responders. Int J Gynaecol Obstetr. 2018;141:327-31.
- [Google Scholar]
- GnRH agonist and hCG (dual trigger) versus hCG trigger for final follicular maturation: a double-blinded, randomized controlled study. Hum Reprod. 2020;35:1648-54.
- [Google Scholar]
- Dual trigger of final oocyte maturation with a combination of GnRH agonist and hCG versus a hCG alone trigger in GnRH antagonist cycle for in vitro fertilization: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2017;218:92-8.
- [Google Scholar]







