Translate this page into:
Impact of day 5 vs. day 6 blastocyst transfer on the pregnancy outcome of frozen-thawed donor recipient cycles
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
To compare pregnancy outcome and early pregnancy loss in day 5 versus day 6 frozen-thawed donor recipient embryo transfer cycles.
Methods:
414 consecutive donor recipient cycles were analysed who had undergone frozen-thawed elective single blastocyst transfer cycles (FET), were non-preimplantation genetic tested (PGT) embryos. High-grade blastocysts were vitrified on day 5 (n = 335) or day 6 (n = 79). Post embryo transfer progesterone supplementation was commenced when endometrial thickness >7 mm was reached after hormone replacement. Frozen blastocysts thawed were assessed for survival, clinical pregnancy, and early pregnancy in both the groups (day 5 [Group A] versus day 6 [Group B]). Statistical analysis was done using chi-square test.
Results:
All parameters like mean age of oocyte donor (25.03 vs. 24.97 years, p = 0.986), endometrial thickness (8.06 mm vs. 8.23 mm, p = 0.961), and embryo survival (97% vs. 98%, p = 0.776) were comparable in both the groups. Clinical pregnancy rate was found to be similar between Group A versus Group B (50.74% vs. 49.36%, p = 0.825, OR 1.05, CI 0.645–1.724). Clinical miscarriage rate in Group B was 33.33% as compared to 17.05% in Group A, p = 0.022, OR 2.43, CI 1.12–5.28) and was statistically significant.
Conclusion:
Pregnancy potential of high‐grade blastocysts frozen on day 5 or day 6 seems to be comparable. Younger age of donors had low expected pregnancy loss rates; however, the miscarriage rate was significantly higher in day 6 embryos. Thus, raises an important question if these cycles would perform better if PGT-A is offered when only day 6 embryos are available for transfer.
Keywords
blastocyst
clinical pregnancy
endometrial thickness
frozen embryo
INTRODUCTION
Embryo cryopreservation have become routine procedures in human-assisted reproductive technologies and have managed to change the landscape in fertility treatment. By 2017, many centers have adopted embryo cryopreservation as their primary IVF therapy and perform few or no fresh embryo transfers. The two important reasons for cryopreservation are to achieve better endometrial receptivity when embryos are transferred in cycles without exposure to ovarian stimulation, and the ability to store the embryos while awaiting the results of preimplantation genetic testing. The practice of cryopreservation for frozen-thawed embryo transfer cycles (FET) is more appropriate for ovarian hyperstimulation syndrome prevention, medical issues, efficacy, or the desirability.[1] Outcome from the several studies that compared fresh and frozen-thawed FET showed significantly higher clinical pregnancy rate with no increase in birth defects or development abnormalities.[2]
However, a substantial increase in rate of multiple births is evident with the development of IVF techniques. To reduce the risk of multiple pregnancy, single embryo transfer (SET) and blastocyst culture have become a recent trend and are associated with significantly higher implantation rates, compared to earlier transfers of cleavage-stage embryos.[3,4] Increased implantation rates maintain good pregnancy rates while transferring fewer embryos, thus reducing the risk of multiple pregnancy.
Identification of characteristics of blastocysts is important to refine the procedure of embryo transfer. Excellent pregnancy rates have been reported with fresh single blastocyst transfers.[3,5] However, some embryos reach blastulation by day 5 and others not until day 6 or even day 7.
There have been various reports in literature about clinical outcomes associated with day 5 and day 6 blastocyst transfers. Results from a meta-analysis study indicated more favorable outcomes for day 5 versus slower developing day 6 vitrified blastocysts.[6] However, when embryos of the same stage are compared, there would appear no significant differences.[6] Contrary to these, few findings showed improved clinical results for day 5 versus day 6 blastocysts at the same developmental stage.[7,8,9]
The prime objective of current study was to compare the pregnancy outcome for SET of day 5 versus day 6 frozen-thawed donor recipient embryo transfer cycles.
MATERIALS AND METHODS
Study Design and Participants
This was a single-center, retrospective cohort analysis of 508 FET cycles from 357 women from 2015 to 2019 at tertiary-assisted conception unit. At the time of oocyte retrieval, recipients were under age 45 and underwent SET of frozen-thawed autologous blastocysts cryopreserved on day 5 or day 6. Embryos that reached an appropriate blastocyst stage were vitrified on day 5 and nonexpanded blastocysts were cultured till day 6. On day 6, fully expanded blastocysts were vitrified.
Embryo grading and cryopreservation
During intracytoplasmic sperm injection, blastocyst formed was assigned grades based on the morphological features of trophectoderm and inner cell mass and on the degree of blastocoele expansion as described by the ASEBIR criteria. Embryos were vitrified with Vitrification Kit (Vitrolife). Good-quality embryo was defined according to the degree of blastocoele expansion and morphological features of the inner cell mass and trophectoderm, as described by Gardner and Schoolcraft. Blastocysts were scored according to their expansion, inner cell mass (ICM), and trophectoderm (TE) development: 1—Early blastocyst, 2—Blastocyst, 3—blastocyst (blastocoele fills the blastocyst), 4—expanded, 5—hatching blastocyst.
ICM: A—Numerous and tightly packed, B—numerous and loosely packed, C—few cells.
TE A—Many cells forming a cohesive epithelium, B—fewer cells organized in a loose epithelium, C—very few cells.
Blastocysts were only selected for freezing if they were at least of grade 3 expansion, grade B of inner cell mass and grade B of trophectoderm on day 5 or day 6 after fertilization prior to cryopreservation.
Analysis and comparison between day 5 and 6 blastocysts was based on classification of 2 groups: Top/good blastocysts (3,4 AA, AB, BA), and fair (3,4 BB). Lower quality blastocysts were not frozen or transferred.
Thawing
All embryos were thawed using the embryo thawing media (vitrolife) and incubated for 2 to 3 hours before transfer. Viable embryos with good cell survival rate and expansion were transferred.
Endometrial preparation and evaluation
Patients were started with administration of oral estradiol valerate, 6 mg in divided doses daily (Progynova) on day 2/3 of menstrual cycle and continued for 10 days, after which the endometrial assessment was performed to assess the endometrial thickness. When endometrial thickness was ≥ 7 mm, embryo transfer was scheduled after 5 completed days of vaginal progesterone in both the groups. Cycles were cancelled if the endometrial thickness had failed to reach 7 mm after a period of 3 weeks of oestradiol valerate.
Luteal support
Progesterone supplementation in the form of micronized progesterone (400 mg twice daily, Gestone, Ferring) was commenced and administered for 5 days before scheduling embryo transfer. Post thawing embryos were assessed immediately for survival, and after 2 to 3 hours for blastocoele reexpansion. In both groups, embryos were transferred on day 6 of progesterone. Pregnancy was detected with serum beta human chorionic gonadotropin (hCG) after 11 days of embryo transfer.
Exclusion criteria in the study was all PGT-A cycles, any submucous fibroid or intramural fibroid ≥3 cm, untreated hydrosalpinx, and adenomyosis.
Clinical pregnancy was defined as the observation of a gestational sac with fetal heart pulsations on ultrasound scanning 2 weeks after the positive pregnancy test. Miscarriage rate included both biochemical and clinical miscarriages.
Data were analyzed by chi square test to compare the variables that included vitrification day, maternal age at the time of oocyte retrieval, endometrial thickness, number of transferred embryos, number of top-quality embryos transferred, implantation rate, and pregnancy loss rate. p < 0.05 was considered statistically significant.
RESULTS
In total, 414 FET were taken and all the embryos were non-preimplantation genetic tested (PGT) embryos. Out of which, 335 in the day 5 (group A) and 79 in the day 6 (group B) were analyzed. [TABLE 1] summarizes the comparison of statistics of group A and group B. Both the groups were comparable regarding average age of oocyte donor (25.03 vs. 24.97 years, p = 0.290), endometrial thickness (8.06 vs. 8.23 mm, p = 0.5164). More good-quality embryos were transferred in the group A (56.74% vs. 39.24%, p = 0.0033), whereas fair-quality embryos were more in group B (25.67% vs. 46.88%, p = 0.0001), but there was no difference between clinical pregnancy rate (50.74% vs. 49.36%, p = 0.825), ongoing pregnancy, and implantation rate (50.1% vs. 46.8%, p = 0.6357). However, we observed a significant change in pregnancy loss rate (17.05 % vs. 33.33%, p = 0.022), as shown in [FIGURE 1].

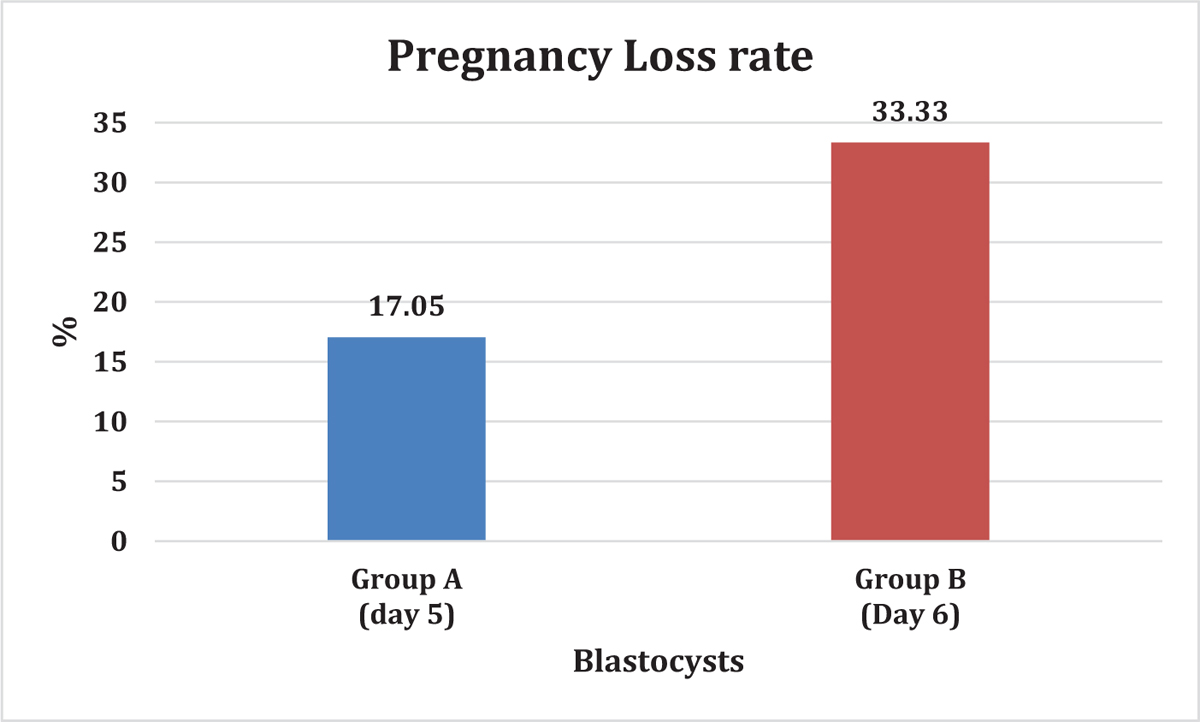
- % pregnancy loss in Group A (Day 5) vs. Group B (Day 6).
DISCUSSION
This study examined whether the use of day 5 or day 6 thawed blastocysts could lead to different treatment outcomes. The data were analyzed retrospectively from a large sample size with thawed blastocyst transfer cycles, in which only high-quality blastocysts were frozen. Blastocyst freezing enhances the efficiency of IVF development by augmenting the chance of pregnancy per oocyte retrieval.[10] Therefore, an understanding of the characteristics of blastocyst development could guide clinical practice to further improve IVF outcomes.[11]
Different studies have shown that in fresh cycle blastocyst transfer on day 5, an increase in pregnancy rate was observed as compared with the blastocyst on day 6.[12,13] These results can be explained as because of delayed embryo growth as well as displaced window of implantation when day 6 embryos are transferred in fresh ET cycle. Shapiro et al. and Khorram et al. have shown that embryos at expanded blastocyst stage and transferred on day 5 have high implantation potential compared to those for which expansion and transfer was done on day 6.[13,14]
In frozen cycle, there are conflicting results of effect of vitrified good-quality day 5 and day 6 blastocyst on pregnancy outcome. Systemic review and meta-analysis of 15 controlled studies[6] comprising slow-freezing and vitrification methods of cryopreservation reported higher clinical pregnancy rate and ongoing pregnancy rate for day 5 transfer compared to day 6 transfers. However, further analysis of these studies where day 5 and day 6 blastocysts had the same morphological quality at the time of freezing showed no difference in clinical pregnancy and ongoing pregnancy rates. Our study too did not find any statistical significance in terms of clinical and biochemical pregnancy rate on day 5 and day 6. Recent meta-analysis by Yi‐Xin Li et al. observed lower implantation rate and lower clinical pregnancy rate in day 6 blastocyst-stage embryo transfer.[15]
However, these findings are not similar in case of SET. Kaye et al. as authors observed no difference in ongoing pregnancy rate, clinical pregnancy rate, live birth rate, or miscarriage rate between day 5 and day 6 frozen and vitrified embryos.[16] Similar to this study, our results also illustrate no significant difference in clinical pregnancy rate, ongoing pregnancy rate, implantation rate, and miscarriage rate in groups of day 5 and day 6.
Literature evidence showed that one of the important reasons for implantation failure is embryo aneuploidy. Recently, Sardana et al.[17] have demonstrated that in PGT-A cycle, irrespective of day of transfer no statistically significant difference was observed in live birth rate following transfer of euploid embryos, whereas day 5 blastocyst transfer was associated with higher live birth rate compared to day 6 blastocysts in untested embryos. In our study, all the embryos were PGT embryos, raising an important question if these cycles would perform better if PGT-A is offered.
Our study shows that day 6 vitrified blastocysts lead to higher miscarriage rate compared to day 5 blastocysts. Similar results were obtained by Xu et al., where they found that day 6 blastocysts transfer were associated with a higher early miscarriage rate.[18] This raises an important question if these cycles would perform better if preimplantation genetic testing is offered where only day 6 embryos are available for transfer.
CONCLUSION
Initial pregnancy potential of high-grade blastocysts frozen on day 5 or day 6 after in-vitro fertilization and replaced in FET donor recipient cycles seems to be comparable. In donor recipient cycles having younger age of donors, SET of day 6 cryopreserved blastocysts resulted in similar clinical pregnancy rate, though significantly higher miscarriage rate.
Ethics Compliance: All ethical approvals required for the study were obtained before the start of the study from Ethical Committee of Sir Ganga Ram Hospital.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Commentary
Blastocyst transfer gives a higher overall pregnancy rate than cleavage stage embryo transfer, hence the blastocyst transfers are more commonly performed in current era. With better understanding of the blastocyst culture, the blastulation rate is sometimes delayed by a day and improved blastocyst expansion is seen on day 6 rather than on day 5. The developmental potential of embryos should not be considered as a negative influence on pregnancy outcomes specially good grade blastocysts.
There is an ongoing debate regarding the best approach to be used with slow developing blastocyst transfer cycles. In such cases, performing fresh transfer irrespective of the expansion stage or deferring transfer and continuing culture until fully developed blastocyst formation is a debate.
In fresh embryo transfer cycle, it has been quite clearly shown that pregnancy rates are significantly higher when blastocyst are transferred in expanded state on the day 5 compared with blastocyst expanded on day 6. On the contrary in thawed blastocyst transfer cycles, same clinical outcome should be expected when transferring day 5 or day 6 blastocyst because of endometrial/embryonic synchronisation due to hormonal priming of endometrial receptivity.
However, the long-term pregnancy outcomes remain still unclear. Some studies have shown higher pregnancy rates of day 5 thawed blastocyst transfer compared with those of the day 6 while others have shown equivalent thawed blastocyst transfer outcomes after day 5 and day 6 cryopreserved blastocyst transfers.
A study by Andrabi et al. from India has shown significant higher live birth rates in day 5 cryopreserved blastocyst than day 6. Biochemical pregnancy rate was higher in this study in the day 6 expanded cryopreserved embryo. Whether the developmental potential was responsible for this increase in biochemical pregnancy rate is debatable. Should all embryos showing delayed blastulation undergo genetic testing before transfer is also another point of discussion and debate too. More research and trials are needed to understand this further.
Dr Puneet Rana Arora, Director, CIFAR, Gurgaon, Haryana, India.
Acknowledgment
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work, and have given final approval for the version to be published. The authors thank Dr Punit Srivastava and Dr Nidhi Gupta of Mediception Science Pvt Ltd (www.mediception.com) for providing medical writing support in the preparation of this manuscript, funded by Merck Specialities Pvt Ltd, India.
REFERENCES
- The future of cryopreservation in assisted reproductive technologies. Front Endocrinol (Lausanne). 2020;11:67.
- [Google Scholar]
- Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96:344-8.
- [Google Scholar]
- Elective single-embryo transfer versus double embryo transfer in in vitro fertilization. N Engl J Med. 2004;351:2392-402.
- [Google Scholar]
- Contribution of embryo cryopreservation to elective single embryo transfer in IVF-ICSI. Reprod Biomed Online. 2006;13:368-75.
- [Google Scholar]
- Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155-8.
- [Google Scholar]
- The influence of delayed blastocyst formation on the outcome of frozen-thawed blastocyst transfer: a systematic review and meta-analysis. Hum Reprod Oxf Engl. 2010;25:1906-15.
- [Google Scholar]
- Clinical pregnancy rate following frozen embryo transfer is higher with blastocysts vitrified on day 5 than on day 6. J Assist Reprod Genet. 2016;33:1553.
- [Google Scholar]
- Growth retardation in human blastocysts increases the incidence of abnormal spindles and decreases implantation potential after vitrification. Hum Reprod Oxf Engl. 2013;28:1528-35.
- [Google Scholar]
- Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles. Fertil Steril. 2016;106:1370-8.
- [Google Scholar]
- High pregnancy rates can be achieved after freezing and thawing human blastocysts. Fertil Steril. 2004;82:1418-27.
- [Google Scholar]
- Blastocyst development rate impacts outcome in cryopreserved blastocyst transfer cycles. Fertil Steril. 2008;90:2138-43.
- [Google Scholar]
- Blastocyst culture after repeated failure of cleavage-stage embryo transfers: a comparison of day 5 and day 6 transfers. Fertil Steril. 2005;83:49-53.
- [Google Scholar]
- A comparison of day 5 and day 6 blastocyst transfers. Fertil Steril. 2001;75:1126-30.
- [Google Scholar]
- Transfer of nonassisted hatched and hatching human blastocysts after in vitro fertilization. Fertil Steril. 2000;74:163-5.
- [Google Scholar]
- Pregnancy outcomes after day 5 versus day 6 blastocyst‐stage embryo transfer: a systematic review and meta‐analysis. J Obstet Gynaecol Res. 2020;46:595-605.
- [Google Scholar]
- Pregnancy rates for single embryo transfer (SET) of day 5 and day 6 blastocysts after cryopreservation by vitrification and slow freeze. J Assist Reprod Genet. 2017;34:913-9.
- [Google Scholar]
- The influence of delayed blastocyst development on the outcome of frozen-thawed transfer of euploid and untested embryos. J Hum Reprod Sci. 2020;13:155-61.
- [Google Scholar]
- D6 blastocyst transfer on day 6 in frozen-thawed cycles should be avoided: a retrospective cohort study. BMC Pregnancy Childbirth. 2020;20:519.
- [Google Scholar]







