Translate this page into:
IUI: Optimizing results, minimizing complications
Address for correspondence: Dr. Archana Mishra, House No.: 26-A, Pocket 4, Mayur Vihar Phase 1, East Delhi, Delhi, India. PIN: 110091. E-mail: drarchanamishra@rediffmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Intrauterine insemination is a procedure in Assisted Reproductive Techniques (ART) where processed and concentrated motile sperms are placed directly into the uterine cavity. Although NICE [2013] recommends In Vitro Fertilisation over intrauterine insemination after 2 years of unprotected intercourse, Intrauterine Insemination (IUI) appears to be a low cost and effective option before proceeding for In Vitro Fertilisation (IVF). Minimal requirement for IUI is patency of at least one fallopian tube. Adequate number of motile sperms should be present in pre wash specimen of semen. At total sperm count 3–5 million, pregnancy rate is less than 1% per cycle. In unexplained infertility there may be undetected subtle functional defects in follicular development, maturation and ovulation like luteinised unruptured follicle, luteal phase defect. Ovarian stimulation increases the number of dominant follicles and improves their quality resulting in improvement in pregnancy rate. Single IUI has benefits of fewer visits, less cost and stress. Evidence suggests no difference in clinical pregnancy rate between single versus double IUI. WHO recommends ejaculatory abstinence of 2–7 days before semen collection (WHO, 2010), for diagnostics and semen preparation. Shorter time interval between processing and insemination leads to less sperm chromatin decondensation & sperm DNA fragmentation and higher Pregnancy Rate. The luteinizing hormone surge can be detected almost 36 hours before ovulation in serum and 24 hours before ovulation in urine. The optimal time interval between human chorionic gonadotropin (hCG) injection and IUI seems to be between 12 and 36 hours; 24 hours after leutinising hormone (LH) Surge. Nature of sperms is the best guide for choice of technique. “Swim up” techniques are recommended in cases of normozoospermia and “Density Gradient” should be chosen in cases of any pathology of semen. The pregnancy rate per cycle is highest in the first three treatment cycles. Most couples show acceptable cumulative ongoing pregnancy rates after six cycles of IUI with ovulation induction. In vitro fertilization should be considered after 3–6 cycles taking all factors into consideration.
Keywords
Infertility
IUI
ovulation induction
pregnancy
INTRODUCTION
Intrauterine insemination is a procedure in assisted reproductive techniques (ART) in which processed and concentrated motile sperms are placed directly into the uterine cavity. Intrauterine insemination allows sperms to bypass potentially hostile cervical factors, thus increasing the number of sperms that may gain access to the uterine cavity. Intravaginal insemination is a low resource alternative for couples who are unable to have or choose to avoid intercourse in men with erectile or ejaculatory dysfunction.[1]
NICE[2] recommends in vitro fertilisation over intrauterine insemination after 2 years of unprotected intercourse. Even after recommendation, almost 96% of fecility clinics of United Kingdom continued to offer Intrauterine Insemination (IUI) before moving on to In Vitro Fertilisation (IVF). One published Randomised Controlised Trial (RCT) revealed that live birth rate was 31% with intrauterine insemination and 9% with expectant management. When ART is considered, we should consider that cost of in vitro fertilization is three to four times of IUI and the initial treatment by IUI will save 20% of couples from moving on to IVF.[3]
Minimal requirement for IUI
Minimal requirement for IUI is patency of at least one fallopian tube. An adequate number of motile sperms should be present in prewash specimen of semen. At total sperm count of 3 to 5 million, pregnancy rate is less than 1% per cycle. At sperm count less than 5 million, IVF with intracytoplasmic sperm injection (ICSI) is a better option. There should be no active cervical, intrauterine, or pelvic infection. IUI may be done with fresh semen or frozen donor sperms. Postwash total motile sperm count should be at least 5 million for achieving successful IUI. Indications of IUI are depicted in Table 1.

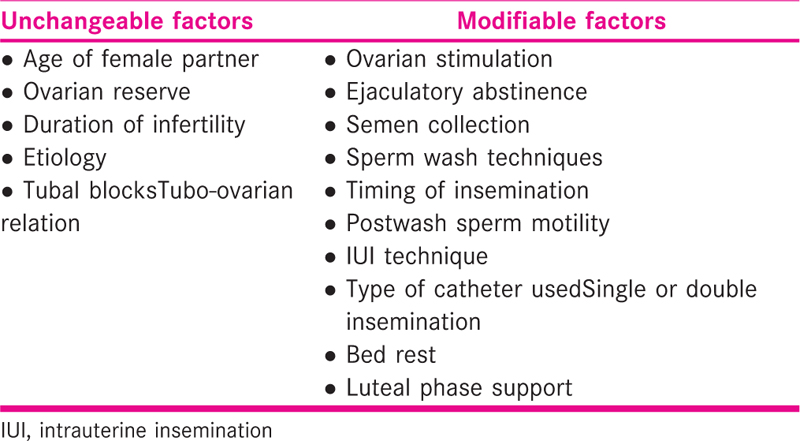
Factors determining the success of intrauterine insemination
A number of factors influence the rate of success of IUI. These factors are listed in Tables 1 and 2.
Rationale of ovulation induction
In unexplained infertility there may be undetected subtle functional defects in follicular development, defects in maturation and ovulation like luteinised unruptured follicle, luteal phase defect. Ovarian stimulation increases the number of dominant follicles and improves their quality resulting in improvement in pregnancy rate.
There is better control over timing through monitoring of ovulation. In couples with unexplained infertility, stimulated cycles with IUI provide better results than natural cycles (odds ratio [OR] 2.14, 95% confidence interval (CI), 1.26–3.6, 25 % vs 14 % pregnancy rates) (Practice Committee Guidelines ASRM 2005).
Optimizing outcomes of intrauterine insemination
In spite of the prevalent use of intrauterine insemination by the fertility experts, there are several dilemmas over superiority of one technique over other. Although a large number of studies and metanalysis are available, interpretation is difficult due to a less number of sample sizes and varying methodology of studies. Clinicians will be benefitted by consensus substantiated by a good level of evidence.
Efficacy of different drugs for ovarian stimulation[4,5]
Drugs that are commonly used for ovarian stimulation are clomiphene citrate, letrozole, and gonadotropins. A few points should be considered before choosing the drug for ovulation induction. Among oral agents, clomiphene citrate has a good ovulation rate of 70% to 80%, but pregnancy rate is lower as it has antiestrogenic effect on endometrium and cervical mucus. Use of letrozole 2.5 mg has been approved by Drug Controller General of India. Letrozole is associated with monofollicular development, does not have antiestrogenic effect on endometrium or cervical mucus, is easy to use, and has a lower risk of multiple pregnancy and ovarian hyper stimulation syndrome (OHSS). In unexplained infertility, clomiphene or gonadotropins will induce multiple follicles as these women have no obvious ovulatory dysfunction, while in polycystic ovarian syndrome (PCOS), letrozole may be preferred initially to avoid multiple pregnancy and OHSS. Gonadotropins seem to be more effective than either oral agent, but their use increases the risk of multiple gestation and OHSS. Both human menopausal gonadotropin (HMG) and recombinant FSH show comparable results when used in unexplained infertility. Both urinary and recombinant follicle stimulating hormone (FSH) give comparable results.[3,4] IUI should be avoided if more than two mature follicles develop on ovarian stimulation (OS).
Doses of gonadotropins for a IUI cycle[5,6]
Dose of gonadotropin 75 IU is ideal for IUI. Doubling the daily dose of gonadotrophins from 75 to 150 IU does not improve outcome, while it significantly increases the rate of multiple pregnancy [evidence level 1a]. Spontaneous leutinising hormone (LH) surges occur frequently in stimulated IUI cycles and might result in lower pregnancy rates [evidence level 2]. Both gonadotrophin-releasing hormone (GnRH) agonists and antagonist are not cost-effective in ovarian stimulation/IUI program [evidence level 1a].
Monitoring of cycle and trigger
Baseline transvaginal ultrasound has to be done on Day 2 to rule out ovarian cyst, endometrial hyperplasia, and antral follicle count. Serial USG monitoring should be done from Day 10 for endometrium pattern, thickness, and growth of follicles. Growth of follicles is approximately 2 mm/day. HCG trigger should be given when dominant follicle is more than 20 mm in clomiphene stimulated cycle and 18 mm in HMG stimulated cycle. With regard to pregnancy rates, there is no significant difference in timing of IUI with human chorionic gonadotropin (hCG) injection or urinary LH surge [evidence level 1a].[7]
Endometrial lining should be more than 8 mm.[8] There is no difference in pregnancy rates with the use of urinary hCG versus recombinant hCG (OR 1.17, 95% CI 0.68 to 2.03). Similarly, there is no difference in outcome with the use of hCG versus GnRH agonist (OR 1.04, 95% CI 0.42 to 2.6, 3 RCTs) or with different doses of hCG (5000 IU vs 10,000 IU).
Double IUI versus single IUI
Single IUI has benefits of fewer visits, less cost, and stress. Evidence suggests no difference in the clinical pregnancy rate between single versus double IUI. Double IUI does not result in higher pregnancy rates compared to single IUI in unexplained subfertility [evidence level 1a]. Double IUI results in higher pregnancy rates compared to single IUI in couples with male factor subfertility (24–44 hours after hCG) [evidence level 1a].[9]
Optimum duration of ejaculatory abstinence
WHO recommends ejaculatory abstinence of 2 to 7 days before semen collection[11] for diagnostics and semen preparation. Longer ejaculatory abstinence has a negative effect on fertilization potential of spermatozoa because of a high amount of reactive oxygen species. Shorter period of ejaculatory abstinence equivalent to 2 to 3 days results in highest pregnancy rates in natural cycle as well as IUI.[10]
Semen collection to processing time
Semen should preferably be collected in a private room near laboratory. When semen is collected at home, it should be delivered to the laboratory within 1 hour after collection (while protecting it from extremes of temperature) (WHO, 2010).[11] No pregnancy is reported if semen is processed more than 60 minutes after being collected.
Storage time after processing
Shorter time interval between processing and insemination leads to less sperm chromatin decondensation and sperm DNA fragmentation and higher pregnancy Rate. Removal of seminal plasma initiates sperm capacitation by changes in the ions in the acrosome. Washed sperms fertilize oocyte only within 2 to 3 hours from preparation time. Higher pregnancy rates are observed when insemination takes place within 90 minutes after semen collection compared to 91 to 120 min and >120 min.[12] The results of different studies are contradictory and different cutoffs of time interval have been taken that make comparison difficult. One of the studies had even shown no difference in ongoing pregnancy rate between immediate insemination versus insemination 1 day after semen processing.[13]
Timing of insemination according to LH surge
The luteinizing hormone surge can be detected almost 36 hours before ovulation in serum and 24 hours before ovulation in urine. Ovum has the capacity to fertilize about 24 hours after ovulation. Sperms are able to fertilize up to 48 hours of intercourse or entrance in genital tract. A systemic review has analyzed that IUI should be based on hospital facilities and convenience of couple, and no particular time of IUI was found superior to another.[14] However, the optimal time interval between hCG injection and IUI seems to be between 12 and 36 hours, 24 hours after LH Surge [evidence level 1b].[7]
Semen preparation techniques
Nature of sperms is the best guide for the choice of a technique. “Swim up” techniques are recommended in cases of normozoospermia and “density gradient” should be chosen in cases of any pathology of semen.[11] Swim up techniques results in lower recovery rates as compared to density gradient centrifugation. It is expected that swim up techniques will result in higher percentage of morphologically normal and motile spermatozoa. The review of literature shows conflicting results in both these parameters.[15,16,17] Swim up technique was found to produce comparable or even lower motility rates and lower percentage of morphologically normal spermatozoa. Consensus of six RCTs done in this field found both the techniques at par and none of it was superior to other.[18]
Temperature during centrifugation and storage
Centrifugation and storage at room temperature at 25°C or at body temperature from 34°C to 37°C does not affect the pregnancy rates.[19] Prolonged storage of processed semen should be avoided.
Minimum number of sperms needed to obtain pregnancy in IUI?
Postwash total motile sperm count should be at least 5 million. Sperm morphology using strict criteria should be followed, that is, at least >4% of sperms should have normal morphology.[11] Total motility in native sample should be at least >30%. If postwash sperm count is <1 million/mL, then no time should be wasted in IUI. Couple should be advised IVF/ICSI straightforward.[1]
Type of cannula used in IUI
A soft tip flexible catheter causes less trauma to the endometrium compared to a hard tip rigid catheter, but it was not found superior in pregnancy rates compared to a firm catheter in various studies.[20,21] Rigid hard tip catheter has an edge for easy cannulation.
Sexual intercourse before and after IUI
Intercourse before performance of IUI increases cervical reservoir of sperms and improves chances of pregnancy. Sperms can remain active up to 72 hours of entrance into female genital tract. Intercourse should not be prohibited even after IUI as it will cover a longer period specially if some follicle ruptures late after HCG trigger as washed sperms are already capacitated and will fertilize only up to 3 to 6 hours after insemination.
Bed rest after IUI
Spermatozoa reach the fallopian tube as soon as 2 minutes after insemination.
After 10 to 15 minutes immobilization subsequent to IUI, there is a significant improvement in cumulative ongoing pregnancy rates and live birth rates.[22,23]
Luteal phase support after IUI
Luteal phase support using vaginal progesterone improves live birth rates in IUI cycles stimulated with low-dose gonadotrophins in couples with unexplained subfertility [evidence level 1b].[24]
Maximum number of cycles
The pregnancy rate per cycle is highest in the first three treatment cycles. Most couples show acceptable cumulative ongoing pregnancy rates after six cycles of IUI with ovarian hyperstimulation (OH).[25] In vitro fertilization should be considered after three to six cycles taking all factors into consideration.
Complications related to intrauterine insemination
Complications may occur in an IUI cycle due to the following factors:
A consequence of ovulation induction
Related to insemination procedure
Obstetric complications
Gynecological complications
Psychological complications
Dealing with difficult cannulation
In some cases, it is difficult to reach up to cervical Os or negotiate the internal Os. Difficult cannulation is an important cause for poor outcome. Few manoeuvres can help a gynecologist to deal with this situation. It is a good practice to perform a mock IUI catheterization before actual insemination. Sometimes the opening of the cervical Os is very tight and cervix is hung up high or deviated to extreme position. Difficulty in visualizing cervix can be overcome by asking the woman to flex and abduct her thighs. In case visualization of cervix is not at all possible, vaginal insemination may be performed. Sometimes intrauterine insemination is difficult due to acute anteversion of uterus. A simple trick is to keep the bladder full and tilt the Cuscos speculum. Traction to the cervix with tenaculum or cervical insemination should be the last resort in these cases. Sometimes the cervix may be stenosed cervix. If the problem was encountered in one attempt of intrauterine insemination, perform hysteroscopy with cervical dilatation in a cycle preceding the next IUI cycle. Firm catheters should be preferred over flexible one. USG guided insemination may be helpful in difficult insemination, but its routine use is not associated with improvement in results and increases the cost.
CONCLUSION
In IUI cycles for unexplained infertility, ovarian stimulation appears to improve outcome in pregnancy rates. IUI gives a higher pregnancy rate when combined with gonadotropins than oral agents. We need to review timing of IUI to optimize pregnancy rates. Five million postwash sperm count appears to be the minimal requirement for successful IUI. Density gradient appears to be the method of choice for sperm wash. IUI may be tried up to six cycles before moving on to IVF/ICSI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Prevention of multiple pregnancies in couples with unexplained or mild male subfertility: randomised controlled trial of in vitro fertilisation with single embryo transfer or in vitro fertilisation in modified natural cycle compared with intrauterine insemination with controlled ovarian hyperstimulation. Br Med J. 2015;350:7771.
- [Google Scholar]
- National Institute for Health and Clinical Excellence. In: Fertility: Assessment and Treatment for People with Fertility Problems. 2013.
- [Google Scholar]
- Intrauterine insemination with ovarian stimulation versus expectant management for unexplained infertility (TUI): a pragmatic, open-label, randomised, controlled, two-centre trial. Lancet. 2018;391:441-50.
- [Google Scholar]
- Ovarian stimulation in infertile women treated with the use of intrauterine insemination: a cohort study from China. Fertil Steril. 2018;109:872-8.
- [Google Scholar]
- Letrozole, gonadotropin, or clomiphene for unexplained infertility. N Engl J Med. 2015;373:1230-40.
- [Google Scholar]
- Effect of gonadotropin types and indications on homologous intrauterine insemination success: a study from 1251 cycles and a review of the literature. Med Res Int. 2017;2017:3512784.
- [Google Scholar]
- What is the optimal follicular size before triggering ovulation in intrauterine insemination cycles with clomiphene citrate or letrozole? An analysis of 988 cycles. Fertil Steril. 2012;97:1089.94-e1-3.
- [Google Scholar]
- The association between follicular size on human chorionic gonadotropin day and pregnancy rate in clomiphene citrate treated polycystic ovary syndrome patients. Gynecol Endocrinol. 2010;26:546-8.
- [Google Scholar]
- Single versus double intrauterine insemination in multi-follicular ovarian hyperstimulation cycles: a randomized trial. Hum Reprod. 2010;25:1684-90.
- [Google Scholar]
- Reactive oxygen species production by the spermatozoa of patients with idiopathic infertility: relationship to seminal plasma antioxidants. J Urol. 1997;157:140-3.
- [Google Scholar]
- Laboratory Manual for the Examination and Processing of Human Semen (5th). Geneva: World Health Organization; 2010.
- Intrauterine insemination (IUI) pregnancy outcome is enhanced by shorter intervals from semen collection to sperm wash, from sperm wash to IUI time, and from semen collection to IUI time. Fertil Steril. 2004;82:1638-47.
- [Google Scholar]
- Intra uterine insemination pregnancy outcome is not affected by a longer time interval between semen processing and insemination. ESHRE Annual Meeting, Helsinki, Finland.
- Synchronised approach for intrauterine insemination in subfertile couples. Cochrane Database Syst Rev (4):CD006942.
- [Google Scholar]
- Nuclear maturity and morphology of human spermatozoa selected by Percoll density gradient centrifugation or swim-up procedure. J Reprod Fertil. 1988;84:551-6.
- [Google Scholar]
- Simultaneous swim-up/swim-down of sperm in assisted reproduction procedures. J Assist Reprod Genet. 1993;10:261-5.
- [Google Scholar]
- A randomized comparison of the methods of sperm preparation for intrauterine insemination. Fertil Steril. 1998;70:574-5.
- [Google Scholar]
- Semen preparation techniques for intrauterine insemination. Cochrane Database Syst Rev (4):CD004507.
- [Google Scholar]
- Semen preparation techniques in intrauterine insemination: a comparison of nontemperature and temperature controlled centrifugation in cases of unexplained infertility. J Hum Reprod Sci. 2013;6:241-4.
- [Google Scholar]
- Soft versus firm catheters for intrauterine insemination. Cochrane Database Syst Rev (11):CD006225.
- [Google Scholar]
- Ultrasonographic endometrial changes after intrauterine insemination: a comparison of two catheters. Fertil Steril. 1997;68:731-4.
- [Google Scholar]
- A randomized study of the effect of 10 minutes of bed rest after intrauterine insemination. Fertil Steril. 2000;74:509-11.
- [Google Scholar]
- Synchronised approach for intrauterine insemination in subfertile couples. Cochrane Database Syst Rev (12):CD006942.
- [Google Scholar]
- Subfertility guidelines in Europe: the quantity and quality of intrauterine insemination guidelines. Hum Reprod. 2006;21:2103-9.
- [Google Scholar]







