Translate this page into:
Comparative study of genital infections in infertile women and in women with recurrent implantation failure
Address for correspondence: Dr Jyoti Pandey, Southend Fertility and IVF Centre, Vasant Vihar, Delhi, India. E-mail: jyo.pandey.88@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pandey J, Malik S, Talwar S. Comparative study of genital infections in infertile women and in women with recurrent implantation failure. Fertil Sci Res 2023;10:105-11.
Abstract
Introduction:
Recurrent implantation failure (RIF) is a clinical entity which refers to a situation when implantation has repeatedly failed to reach a stage when there is no objective evidence of a pregnancy. There may be various causes of implantation failure, that is, quality of oocyte and sperms, endometrial receptivity, and few recent studies have suggested that genital infection may be the cause of RIF.
Material and methods:
A total of 114 women were enrolled in the study and were divided in two Study group • (Group 1) − Women with 2 to 3 IVF implantation failure • Control (Group 2) − Women presenting with other cause of infertility. After initial work up, vaginal, endocervical swab, and endometrial biopsy were taken from both the groups on day 26 to day 28 of the menses and were sent to the same laboratory. Vaginal swab were examined for Gram's stain, and culture. Cervical swab for Pap smear and endometrial biopsy were examined for culture, histopathology, and immunohistochemistry.
Results:
The incidence of infertility was more common in the age group of 31 to 40 years and incidence of RIF was more after 40 years. Most common factor in our study causing RIF was tubal factor followed by Decreased ovarian reserve (DOR). Incidence of CD138 positivity was more common in women with RIF compared to women with other factors of infertility.
Conclusion:
Our study showed that CD138 positive status is more common in women presenting with RIF and it is also associated with abnormal histopathological findings of endometrium. To evaluate its treatment and effect on future fertility studies with larger sample size is required.
Keywords
CD138 (Syndecan)
chronic endometritis (CE)
genital infections
recurrent implantation failure (RIF)
INTRODUCTION
Infertility has become a global health issue as the incidence of infertility ranges from 8% to 12% in reproductive age group couples.[1] This has lead to increased demand of assisted reproductive technologies (ARTs).
Success of ART depends on many factors which include factors related to embryo and endometrium. Hence, implantation is the critical step in success of ART. Implantation involves steps like apposition, rolling, adhesion, and invasion.
Successful implantation depends on several factors like quality of embryo, intact endometrial cavity and lining, and receptivity of endometrium.
Recurrent implantation failure (RIF) is defined as a situation when implantation has repeatedly failed to reach a stage when there is no objective evidence of a pregnancy, that is, negative urine or blood pregnancy test (human chorionic gonadotrophin, HCG). Currently, there is no universally accepted criteria for RIF (Tables 1-3).
| Factors | Patients with RIF (n = 57) | Patients with Infertility (n = 57) | Total | P value |
|---|---|---|---|---|
| Unexplained | 1 (1.75%) | 7 (12.28%) | 8 (7.02%) | 0.061* |
| Diminished ovarian reserve | 16 (28.07%) | 9 (15.79%) | 25 (21.93%) | 0.113† |
| H/O PID | 16 (28.07%) | 16 (28.07%) | 32 (28.07%) | 1† |
| Tubal | 21 (36.84%) | 13 (22.81%) | 34 (29.82%) | 0.101† |
| Fibroid | 1 (1.75%) | 2 (3.51%) | 3 (2.63%) | 1* |
| Endometriosis | 5 (8.77%) | 4 (7.02%) | 9 (7.89%) | 1* |
| PCOS | 12(21.05%) | 14 (24.56%) | 26 (22.81%) | 0.655† |
| Polyp | 1 (1.75%) | 0 (0%) | 1 (0.88%) | 1* |
| Secondary | 1 (1.75%) | 0 (0%) | 1 (0.88%) | 1* |
| RPL | 1 (1.75%) | 0 (0%) | 1 (0.88%) | 1* |
| Hyperprolactinemia | 0 (0%) | 1 (1.75%) | 1 (0.88%) | 1* |
| Obesity | 0 (0%) | 1 (1.75%) | 1 (0.88%) | 1* |
*Fisher's exact test, †Chi square test
| Cervical Findings | Patients with RIF (n = 57) | Patients with Infertility (n = 57) | Total | P value |
|---|---|---|---|---|
| Gram | ||||
| Negative | 55 (96.49%) | 56 (98.25%) | 111 (97.37%) | 1* |
| Yeast | 1 (1.75%) | 1 (1.75%) | 2 (1.75%) | |
| Yeast, vaginosis | 1 (1.75%) | 0 (0%) | 1 (0.88%) | |
| Aero | ||||
| Sterile | 56 (98.25%) | 55 (96.49%) | 111 (97.37%) | 1* |
| Klebsiella | 1 (1.75%) | 2 (3.51%) | 3 (2.63%) | |
| Anero | ||||
| Sterile | 57 (100%) | 56 (98.25%) | 113 (99.12%) | 1* |
| Trichomonas | 0 (0%) | 1 (1.75%) | 1 (0.88%) | |
| Vaginal Findings | Patients with RIF (n = 57) | Patients with Infertility (n = 57) | Total | P value |
|---|---|---|---|---|
| Gram | ||||
| Negative | 55 (96.49%) | 55 (96.49%) | 110 (96.49%) | 1* |
| Vaginosis | 0 (0%) | 1 (1.75%) | 1 (0.88%) | |
| Yeast | 2 (3.51%) | 1 (1.75%) | 3 (2.63%) | |
| Aero | ||||
| Sterile | 56 (98.25%) | 54 (94.74%) | 110 (96.49%) | 0.618* |
| Klebsiella | 1 (1.75%) | 2 (3.51%) | 3 (2.63%) | |
| Anero | ||||
| Sterile | 57 (100%) | 55 (96.49%) | 1 12 (98.24%) | 1* |
| Trichomoniasis | 0 (0%) | 2 (3.51%) | 2 (1.75%) | |
The investigation and management of RIF usually focuses on the quality of the embryo and endometrial receptivity. In recent studies, the focus is also on importance of maternal systemic diseases, such as thyroid, thrombophilia, and immunological disorders.[2,3,4]
Lactobacillus is the dominant genera in the vaginal microbiome of healthy females, and its key metabolites, that is, lactic acid can maintain the acidic and anaerobic vaginal environment and protect it from pathogen infection.
Gram-negative bacteria secrete endotoxins and these endotoxins if present in high levels induce inflammatory response via TH1 cells, which render endometrium unfavorable for implantation. Endometritis is a sequel of an inflammatory reaction due toTH1 cells. In Chronic endometritis (CE), usually no causative organism is identified, whereas in acute endometritis causative organism may be isolated.
The subclinical infection and inflammation in the endometrium are important etiological factors which affect fertility, and cause implantation failure, spontaneous abortion, preterm birth, and it needs adequate intervention.[5] Hence, we decided to compare the prevalence of genital infection in women with RIF and in other women with infertility.
MATERIAL AND METHODS
It was a case control study conducted at Southend fertility centre from November 2021 to July 2022. The study was conducted after taking the ethical clearance from the ethical committee. A total of 114 women were enrolled in the study and were divided in two groups.
Inclusion Criteria
For cases (Group 1) − Women with 2 to 3 IVF/implantation failure (<45 years of age)
For control (Group 2) − Women presenting with other causes of infertility OR presenting to us for first time.
Exclusion Criteria
Women with H/O antibiotic treatment in the month preceding the biopsy,
Women with uterine malformation, or cavity-deforming fibroids or polyps,
Women with unexplained uterine bleeding,
Couples with male factor of infertility were excluded from the study.
Initial work-up of the patient involved obtaining detailed history, and sending the base line investigations like BG, CBC, LFT, KFT, RBS, HbA1C, thyroid function tests, viral markers, and TORCH infections.
All the patients underwent basic gynecological examination, that is, per speculum examination, per vaginal examination followed by baseline pelvic USG to rule out any structural cause of RIF.
Sample collection: Samples were taken on day 26 to day 28 of the menses in women having regular menstrual cycle, and in women with irregular menstrual cycles swabs were taken on day 26 to day 28 of the cycle and endometrial biopsy was taken on day 1 of the cycle. Pipelle's endometrial curette was used for endometrial biopsy.
Vaginal and cervical swabs were sent for gram stain and culture. Endometrial biopsy was sent for culture histopathological examiation and immune histochemistry with CD 138.
RESULTS
Our study shows that most of our patients in both the groups fall in the age group of 31 to 40 years. But incidence of RIF is higher after age of 40 years and the difference is statistically significant. (Fig. 1)
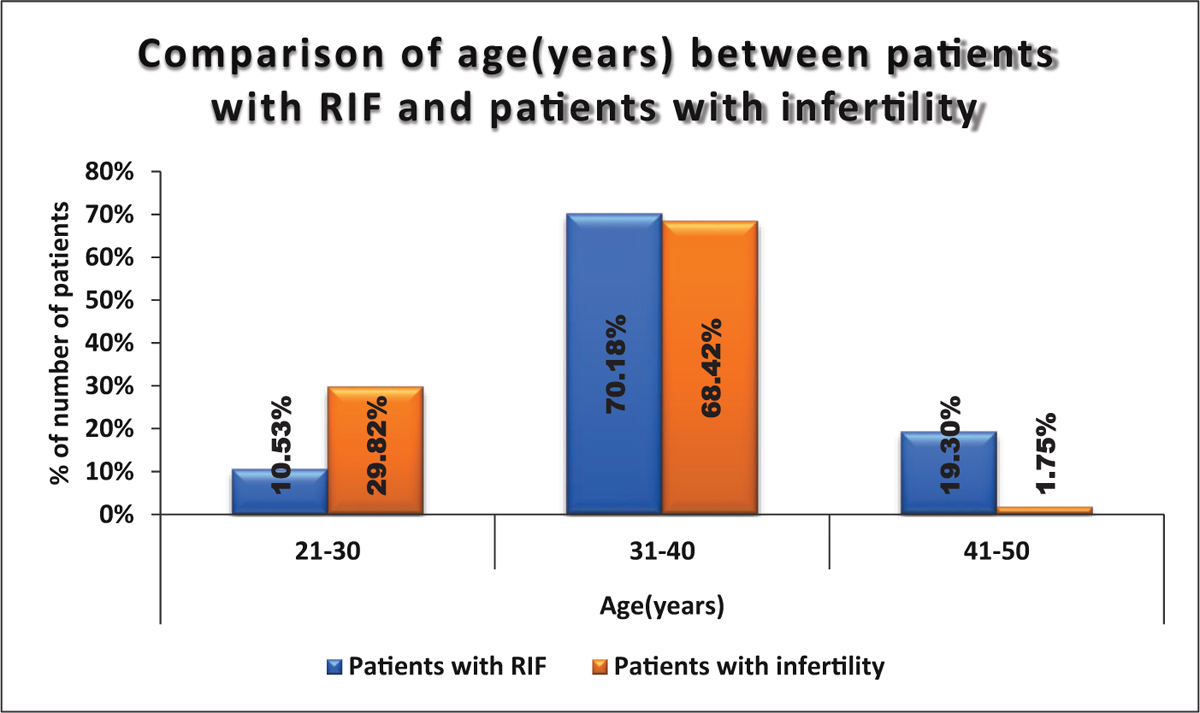
- Comparison of age (years) between patients with RIF and patients with infertility.
Comparison of CD 138 results between the two groups shown in Fig. 2. Comparison of factors of infertility shows that incidence of decreased ovarian reserve and tubal factor was more in women with RIF compared to other patients. Although the difference is not statistically significant. (Table 1)
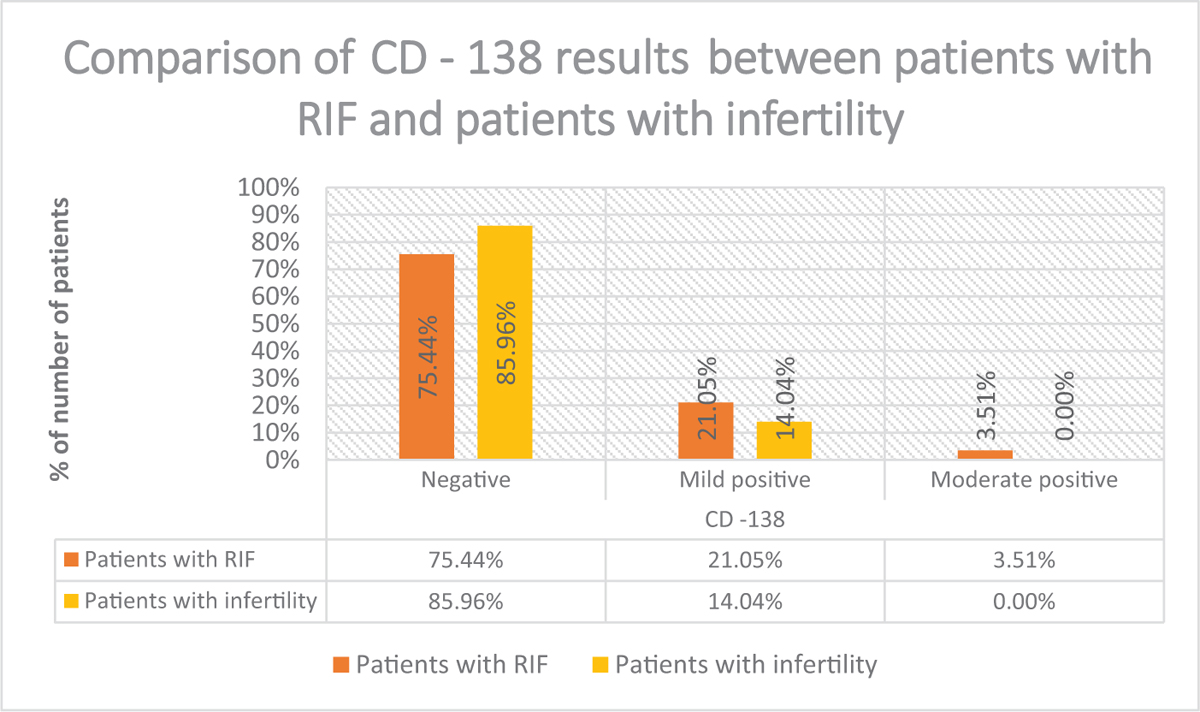
- Comparison of CD138 results between patients with RIF and patients with infertility.
Comparison of vaginal and cervical culture reports was not significantly different (Tables 2 and 3) Association of different histopatholoical patterns with CD 138 positivity are seen in Fig 3 and Table 4.
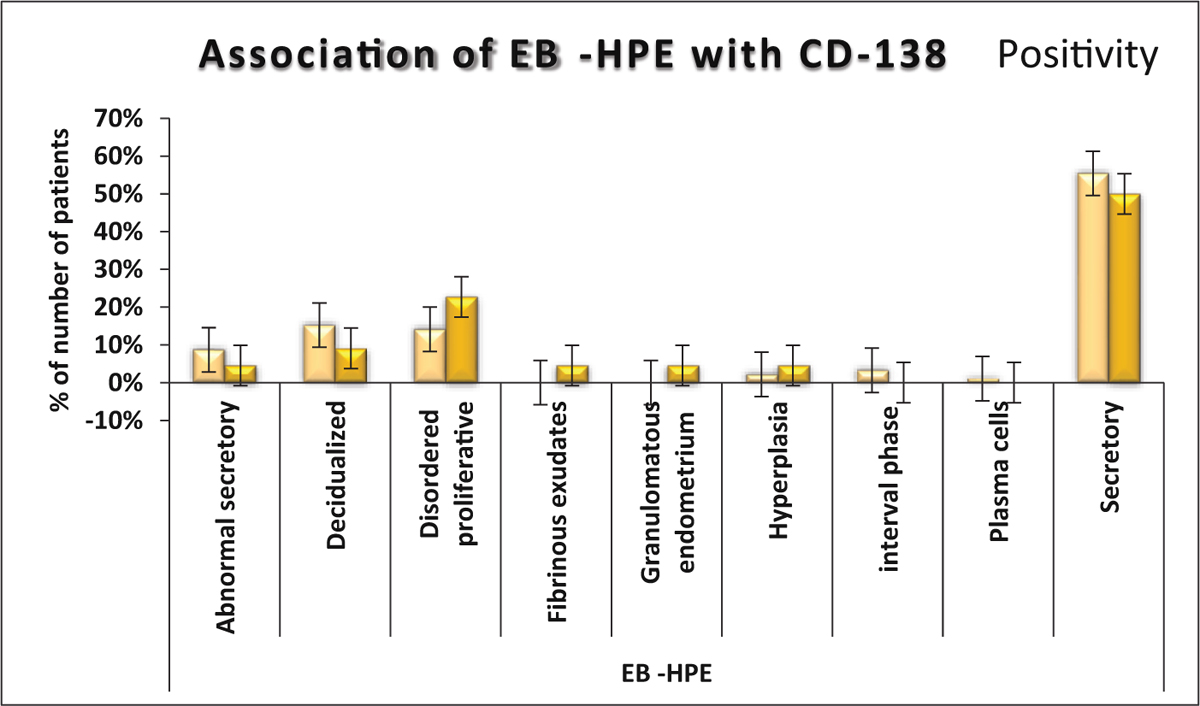
- Association of EB −HPE with CD138.
| EB -HPE | Negative (n = 92) | Positive (n = 22) | Total | P value |
|---|---|---|---|---|
| Abnormal secretory | 8 (8.70%) | 1 (4.55%) | 9 (7.89%) | 0.238* |
| Decidualized | 14 (15.22%) | 2 (9.09%) | 16 (14.04%) | |
| Disordered proliferative | 13 (14.13%) | 5 (22.73%) | 18 (15.79%) | |
| Fibrinous exudates | 0 (0%) | 1 (4.55%) | 1 (0.88%) | |
| Granulomatous endometrium | 0(0%) | 1(4.55%) | 1(0.88%) | |
| Hyperplasia | 2(2.17%) | 1(4.55%) | 3(2.63%) | |
| Interval phase | 3(3.26%) | 0(0%) | 3(2.63%) | |
| Plasma cells | 1(1.09%) | 0(0%) | 1(0.88%) | |
| Secretory | 51(55.43%) | 11(50%) | 62(54.39%) | |
| Total | 92(100%) | 22(100%) | 114(100%) |
DISCUSSION
The female reproductive tract is well known to have an active microbiome. In fact, with more than 20 studies completed, most of the studies found that there is a small but active microbiome in the uterine cavity. Hence, in women undergoing ART cycles it is important to have a healthy reproductive microbiota in addition to treatment of other factors for optimum success of ART cycle.
The mean age in our study was 35.86 ± 4.29 years. The mean age in study done by T. Ichiyama et al. was 38.36 ± 4.2 years[6] and study by Pierre-Emmanuel Bouet et al. showed that mean age of patients with RIF was 36.3 ± 4.2 years.[7] These studies suggest that incidence of RIF increases after 35 years of age. As age increases, the oxidative stress to oocytes increases, leading to decrease in the number and quality of oocyte due to increased chromosomal dysjunction, decrease in mitochondrial membrane potential, and increase in mitochondrial DNA damage.[8]
Our study showed that the most common factor was tubal (tubal block + hydrosalpinx) (34%), history of pelvic inflammatory diseases (PID) (32%), Polycystic ovarian syndrome (PCOS) (26%), followed by decreased ovarian reserve (DOR) (25%). The tubal factor was more common in RIF group compared to other group. Incidence of history of PID was equal in both the groups. It's a well-known fact that history of PID may cause tubal block or hydrosalpinx if not treated adequately. The study by Pierre-Emmanuel Bouet et al. suggested that most common cause of RIF was male factor (32%) followed by unexplained (20%) and then PCOS and endometriosis (6%) each.[9]
Mechanism by which tubal inflammation causes RIF may be (a) direct toxic effect on the embryo,[10] (b) impaired blood flow in subendometrial and endometrial tissues,[11] (c) impaired receptivity of the endometrium,[12] (d) abnormality in the formation of the endometrium,[13] and (e) impaired contact between the embryo and the endometrial surface by direct mechanical effects.[8] The similar finding was suggested by C Coghlan et al. and they also suggested that hydrosalpinges if present should be removed to improve implantation and pregnancy rates.[14] PCOS and DOR are associated with increased oxidative stress and hence poor quality of oocytes, which affects endometrial receptivity as well. All the patients in both the groups had inflammatory Pap smear with evidence of dense inflammatory infiltrates. This finding suggests that all the infertility patients should be evaluated for the same and if required adequate treatment should be given followed by repeat Pap smear.
Our study showed that there was no significant difference in the culture report of women with RIF and in other women with infertility. Our result is consistent with study done by A. Bernabeu et al.[15]
Treatment of women with vaginitis
Women diagnosed with trichomoniasis were treated with tab metronidazole 400 mg twice daily × 7 days.
Women diagnosed with Klebsiella vaginitis were treated with tab cefixime 200 mg twice daily × 7 days.
Women diagnosed with candidiasis were treated with three doses of oral fluconazole 150 mg every 3 days.
All the women who were diagnosed with positive culture reports were given vaginal prebiotics in addition to oral antimicrobial agents prior to starting infertility treatment.
Our results suggest that there is increased incidence of abnormal histopathological finding or out-of-phase endometrium in women with RIF, which includes disordered proliferative endometrium, presence of plasma cell, granulomatous inflammation, and interval-phase endometrium which may cause RIF.
CD138 (Syndecan-1) is a surface proteoglycan which facilitates cell-to-cell adhesion, cell to extracellular matrix adhesion, cell proliferation, and migration.[10] It is used to detect plasma cells in flow cytometry and it can detect both malignant and benign plasma cells in paraffin-embedded bone marrow biopsies.[16]
Immunohistochemical staining with CD138 increases the plasma cell detection, decreases the time consumption looking for plasma cells, and also it is more sensitive to detect chronic endometritis.
Plasma cells have characteristic “clock-face chromatin in an eccentrically placed nucleus with a perinuclear halo” appearance. Identification of plasma cells can be difficult in early proliferative endometrium or late menstrual endometrium, with monocytic inflammatory infiltrates, ample stromal mitoses, stromal cell proliferation, or a marked predecidual reaction in a late secretory endometrium.[11,12]
It should be noted that mere presence of CD138 positivity should not be considered chronic endometritis because there may be limited number of plasma cells without an inflammatory process.[13] The presence of a significant amount of plasma cell has to be considered for confirmation of chronic endometritis. In our study, the reference values were as follows:
Healthy: ≤ 6 CD138 positive plasma cells/HPF
Mild CE: 7–12 CD138 positive plasma cells/HPF
Moderate CE: 13–30 CD138 positive plasma cells/HPF
Severe CE: >30 CD138 positive plasma cells/HPF
The study by K Kutora et al. which analysed the effect of chronic endometritis on endometrial receptivity defined CD138 positivity as >5 CD138 positive cells/HPF. Our study had a more strict criteria for CD138 positivity status. Our study showed that positivity rate of CD138 was 24.5% in RIF group and 14% in control group. Our study has slightly higher incidence of CD138 positivity as compared to study done by Bouet et al., which showed 14% prevalence of CE using histological evaluation in RIF.[7]
Treatment of CD138 Positive Women
All women with positive CD138 reports were treated with antibiotics to cover both gram-positive and gram-negative organisms, that is, tab ofloxacin 400 mg twice daily × 21 days and tab metronidazole 400 mg twice daily × 7 days.
In our study, 22 women who had positive CD138, 5 women, that is, 22.73% had disordered proliferative endometrium, whereas out of 92 women with negative CD138, only 13 women, that is, 14% women had disordered proliferative endometrium. This shows that there is corelation of CD138 positivity status and phase of receptivity of endometrium. This finding is consistent with the study done by K Kuroda et al, which suggested that CE inhibits optimal decidual transformation of the endometrium and shifts or removes the WOI (window of implantation), leading to acquired implantation failure.[17]
CONCLUSION
Chronic endometritis is known to be one of the important causes of RIF; however, the lack of a universally standard definition for RIF indirectly leads to selection bias of patients who should be investigated for CE. Many patients in infertility group were tested before the start of ART cycle if they had any history of tubal disease or PID or after first failed fresh or frozen embryo transfer. Some percentage of these patients may also behave as RIF patients in subsequent IVF cycles. Our study shows correlation between chronic endometritis and disordered proliferative endometrium, hence in women with history of RIF infection should also be ruled out along with other causes of hormonal imbalances.
Financial support and sponsorship
Nil.
COMMENTARY
Commentary on “Comparative study of genital infections in infertile women and in women with recurrent implantation failure”
The authors[18] are to be commended for tackling the subject of recurrent implantation failure (RIF), which is an area of practice where clinicians are unsure as to the correct management. The reasons for this lack of clarity are several—for a start there is no clear consensus on the definition of RIF, with the draft European Society of Human Reproduction and Embryology (ESHRE) guideline talking about an “individualized” definition rather than a “one-size-fits-all” approach (https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Guidelines-in-development/RIF).[19] The authors chose “Women with two to three in vitro fertilization (IVF)/implantation failure,” which is a reasonable definition. Furthermore, patients who present in this way are almost certainly a heterogenous group, and a number of mechanisms can potentially explain the lack of implantation in individual cycles. Much attention has focused on the chromosomal status of the embryo, and it may be the single most important factor determining lack of success. A recent study showed that the incidence of failed treatment was <5% after three transfers of euploid blastocysts into a morphologically normal endometrium.[20] The authors have chosen to focus on the endometrium as a potential cause for RIF in their patients, rather than taking a more holistic approach. This is understandable given the limited use of preimplantation genetic testing for aneuploidy (for valid reasons), but this has the effect of diluting their findings because a high proportion of failed implantation in their patients could have been due to embryos aneuploidy. With the limitations, they do a useful piece of work in demonstrating a higher frequency of immunohistochemical evidence of plasma cell infiltration in women with RIF compared to a general population with infertility. Specifically, they found that CD138, a plasma cell–specific stain was more prevalent in the RIF group, and its presence correlated with histological abnormalities. The value of this study is in focusing the attention of clinicians on endometrial factors and their management in the context of RIF.
Mathur Raj, Manchester NHS Foundation Trust, Manchester, UK.
Conflicts of interest
There are no conflicts of Interests among the authors.
REFERENCES
- Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. 2015. Hum Reprod Update. 21:411-26.
- [CrossRef] [PubMed] [Google Scholar]
- The role of thrombophilia and thyroid autoimmunity in unexplained infertility, implantation failure and recurrent spontaneous abortion. 2008. Hum Reprod. 23:278-284.
- [CrossRef] [PubMed] [Google Scholar]
- Investigation and current management of recurrent IVF treatment failure in the UK. 2005. BJOG. 112:773-80.
- [CrossRef] [PubMed] [Google Scholar]
- Future directions of failed implantation and recurrent miscarriage research. 2006. Reprod Biomed Online. 13:71-83.
- [CrossRef] [PubMed] [Google Scholar]
- Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril. 2004;82:799-804.
- [CrossRef] [PubMed] [Google Scholar]
- Relevance of assessing the uterine microbiota in infertility. Fertil Steril. 2018;110:337-43. doi: 10.10/j.fertnstert.2018.04.041. Review
- [CrossRef] [PubMed] [Google Scholar]
- Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V 1–2 region of the 16S rRNA gene. Peer J. 2016;4:e1602.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence of hydrosalpinges after transvaginal aspiration of tubal fluid in an IVF cycle with development of a serometra. Hum Reprod. 1997;12:703-5.
- [CrossRef] [PubMed] [Google Scholar]
- , Hachem HE, Monceau E, Gariepy G, Kadoch I-J., Sylvestre C. Chronic endometritis in women with recurrent pregnancy loss and recuurent implantation failure: prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil Steril. 2016;105:106-10.
- [CrossRef] [PubMed] [Google Scholar]
- Hydrosalpinx fluid has embryotoxic effects on murine embryogenesis: a case for prophylactic salpingectomy. Fertil Steril. 1996;66:851-3.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of endometrial and subendometrial blood flows among patients with and without hydrosalpinx shown on scanning during in vitro fertilization treatment. Fertil Steril. 2006;85:333-8.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of hydrosalpinx on markers of endometrial receptivity. Semin Reprod Med. 2007;25:476-82.
- [CrossRef] [PubMed] [Google Scholar]
- The significance of hydrosalpinx in in vitro fertilization. Fertil Steril. 1998;69:373-84.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent implantation failure: definition and management. 2014. Reprod Biomed Online. 28:14-38.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of the vaginal microbiome on the pregnancy rate in women receiving assisted reproductive treatment. J Assist Reprod Genet. 2019;36:2111-9. doi: 10.1007/s10815-019-01564-0. Epub 2019 Aug 24. PMID: 31446545; PMCID: PMC6823330
- [CrossRef] [PubMed] [Google Scholar]
- Management of infertility in women over 40. Maturitas. 2014;78:17-21. doi: 10.1016/j.maturitas.2014.02.014. Epub 2014 Mar 5. PMID: 24679892
- [CrossRef] [PubMed] [Google Scholar]
- Impact of chronic endometritis on endometrial receptivity analysis results and pregnancy outcomes. 2020. Immun Inflamm Dis. 8:650-8.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative study of genital infections in infertile women and in women with recurrent implantation failure. Fertility Science research Journal 2023 (Ahead of publication)
- [Google Scholar]
- Recurrent implantation failure: how common is it? Curr Opin Obstet Gynecol. 2021;33:207-12.
- [CrossRef] [PubMed] [Google Scholar]







