Translate this page into:
Assessment of oxidative stress in follicular fluid of infertile women undergoing IVF procedure
Address for correspondence: Shreya Nautiyal, 5/3 Balbir Road, Dalanwala, Dehradun, Uttrakhand-248001, India. E-mail: shreyanautiyal11@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Oxidative stress (OS) plays an important role in biological processes of reproduction, and yet there is no accurate indicator to measure it. The objective of the study was to correlate the effect of ROS (reactive oxygen species) on embryo quality and pregnancy outcome. This was achieved by measuring levels of ROS present in granulosa cells and follicular fluid (FF) of women undergoing IVF (in vitro fertilization). ROS in FF is measured using thiobarbituric acid reactive substances assay kit (Abcam) and granulosa cells were measured with the help of flow cytometry. The results showed that high levels of FF malondialdehyde were found in nonpregnant women as compared to pregnant group and were found to be significant (P < 0.0001) and ROS in granulosa cells showed the same trend but was found to be statistically nonsignificant (P = 0.1658). It was also seen that the number of metaphase II oocytes were affected neither by ROS in granulosa cells nor in FF (P > 0.05) but had an impact on the quality of embryos (P < 0.05). Hence, we can conclude that OS is a real threat to embryos especially in vitro and it has a negative impact on IVF outcomes. Imbalance in ROS can lead to poor fertilization rate, implantation, and pregnancy outcomes and ROS levels in FF and granulosa cells can be taken as a potential marker to detect IVF treatment outcomes.
Keywords
Follicular fluid
oxidative stress
reactive oxygen species
INTRODUCTION
In vitro fertilization (IVF) is an accepted treatment for infertility, but unfortunately the success rate of this procedure in an average rate/cycle is approximately 30% to 40%.[1,2] There are various reasons for IVF failure, and oxidative stress (OS) is regarded as an important factor[3]; however, there has not been much research on the effects of ROS in female infertility.[4,5,6].
Follicular fluid (FF) creates a suitable environment for the oocyte development and increased ROS activity in FF can be lethal for embryo formation. Therefore, the aim of this study was to measure the levels of intracellular ROS in granulosa cells and lipid peroxidation in the FF of women during IVF and to assess their impact on outcome of IVF.[7]
MATERIAL AND METHODS
Granulosa cells and FF samples from patients/donors undergoing ovum retrieval at Ridge IVF, Delhi from December 2019 to February, 2020 were collected according to the inclusion criteria given below.
Inclusion criteria
Female age: 23 to 38 years.
FF samples containing at least one mature oocyte and having normal morphology were included.
Only females whose partners’ semen sample was normozoospermic were taken.
Absence of any metabolic or endocrine system-associated diseases (e.g., hyperprolactinemia and thyroid dysfunction) and no history of cancer.
Exclusion criteria
Female age: <23 or >38 years.
FF contaminated with blood or having immature oocyte/no oocytes were excluded.
Females whose partner had severe male factor (teratozoospermia).
Sample size calculation
Descriptive statistics were done using Statistical Package for Social Sciences (SPSS) version 17.0 software. For tests of association using bivariate correlations, a moderate correlation was made. To detect a moderate correlation (r = 0.30), a sample of 49 analyzable subjects will provide 80% power to discover that the correlation is significantly different from there being no correlation (i.e., the correlation would be zero) at the 0.05 level.
Patients gave their written informed consent and did not receive any monetary compensation for participating in the study. Basal follicle stimulating hormone (FSH), luteinizing hormone, and estradiol levels were tested on cycle day 2 of a spontaneous menstrual cycle prior to ovarian stimulation. The ovarian stimulation prior to ovum pickup (OPU) began with 150 to 225 International unit (IU) of recombinant FSH (Gonal-F®; Merck Serono; Darmstadt, Germany) from day 2 of their menstrual cycle and the Gonadotropin releasing hormone (GnRH) antagonist (Cetrotide; Merck Serono, Darmstadt, Germany) or agonist (Lupride, Sun Pharma) protocol was used. Regular follicular monitoring was done till optimum follicular size was achieved. The trigger was given with either recombinant human chorionic gonadotropin (hCG) (Ovitrelle®, Merck Serono) or agonist (lupride 1 mg s/c) and retrieval was done 34 to 36 hours post trigger.
FF collection and processing
FF aspiration under OPU procedure was done transvaginally with the help of a transvaginal ultrasound probe. An oocyte aspiration needle (Cook) was used with a pump (Craft suction unit, Rocket medical) for oocyte retrieval. Once the pickup was done, the oocytes were placed into the culture media, while FF was collected in 15 ml Becton Dickinson (BD) conical tubes. FF was centrifuged and the supernatant was stored at −20°C for further MDA (malondialdehyde) analysis. The FF of immature oocytes (germinal vesicle or metaphase I) were not considered for the study. Visual inspection was done to observe blood contamination and samples that looked cloudy or blood stained were rejected. Only uncontaminated FF that had minimum stain with blood was used for analysis. Samples that were without oocytes or contaminated were rejected.
Lipid peroxidation assay
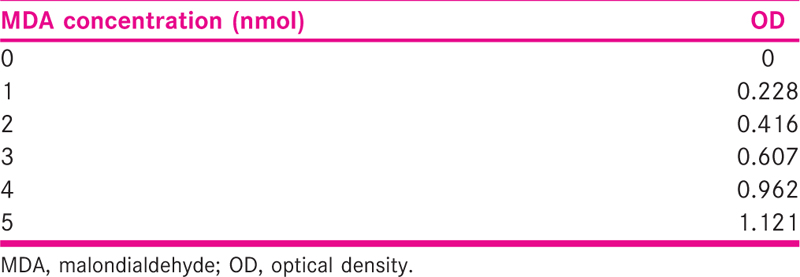
FF TBARS (thiobarbituric acid reactive substance) levels were measured using a TBARS assay kit (Abcam). The product that is produced by reacting MDA with thiobarbituric acid after 1 hour at 95°C was estimated using a spectrofluorimeter with 530 nm being the excitation level and the emission level being 550 nm. TBARS was expressed in terms of MDA equivalent (nmol/mL). A standard curve was first generated using the MDA standard with the kit.
The collected FF samples were stored at −20°C in the laboratory. The TBA (thiobutiric acid) reactive test was performed to determine the MDA levels in the FF. This test detects MDA that responds to TBA, which is therefore known as TBARS. FF, which responded to a combination of 15% TCA (trichloroacetic acid), TBA, and HCl reactive, was incubated in boiling water for 1 hour and later cooled. The liquid was then centrifuged at 3500 rpm for 10 minutes and the absorbance values were measured on the Molecular Device F5 plate reader at 535 nm.
ROS assessment by flow cytometry analysis
Granulosa cells were centrifuged and the supernatant was not taken. The cells were washed twice with PBS (phosphate buffer saline). To determine the level of intracellular ROS generation, these cells were incubated with dye, that is, H2DCFDA (2’,7’-dichlorodihydrofluorescein diacetate; 2.5 μM) in RPMI medium without serum and phenol red for 15 min at 37°C. H2DCFDA is a chemically reduced form of fluorescein. The 20,70-dichlorofluorescein (DCF) fluorescent probe is particularly sensitive to hydrogen peroxide, peroxynitrite, and hydroxyl radicals. Superoxide anions can also lead to H2DCFDA oxidation. Although other more specialized ROS probes are present, H2DCFDA remains among the most effective indicators of cellular OS and remains the most effective standard for ROS measurement. After labelling, the cells were washed and resuspended with PBS and immediately measured using an Acuri flow cytometer (Becton-Dickinson, USA). Granulosa cells suspension was used and the machine was programmed to read 10,000 cells. The fluorescence properties of 10,000 cells were collected for a single analysis. The gates were measured using the forward scatter and side scatter properties of cell populations. The viability of these cells was checked by flow cytometry using propidium iodide staining and was found to exceed 95%. The data were analysed using the Becton Dickinson–fluorescence activated cell sorting (BD FACS) Diva software (Becton-Dickinson, USA).
ROS production was estimated by mean fluroisothynocyanite (FITC) fluorescence intensities and then expressed as a percentage of the ROS that the granulosa cells produce. The ability of granulosa cells to provide oxidative stress by dividing the percentage of ROS producing granulosa cells after H2DCFDA stimulation by the percentage of granulosa cells that produce ROS under basic conditions.
RESULTS
The study group consisted of females with mean age 32.3 ± 4.8 years, mean body mass index (BMI) 61.98 ± 10.45, and having a duration of infertility of 3.6 ± 2.1 years. Antagonist protocol was followed in majority of the cases (94%). The two groups of patients, pregnancy positive (group I) and pregnancy negative (group II), were compared in terms of the levels of ROS in their granulosa cells and FF.
The mean ROS percentage in granulosa cells of pregnant women was 51 ± 18.73, which was slightly less compared to nonpregnant women whose mean ROS percentage was 60 ± 19.02. But this was statistically nonsignificant [Figures 1 and 2].

- Standard curve.
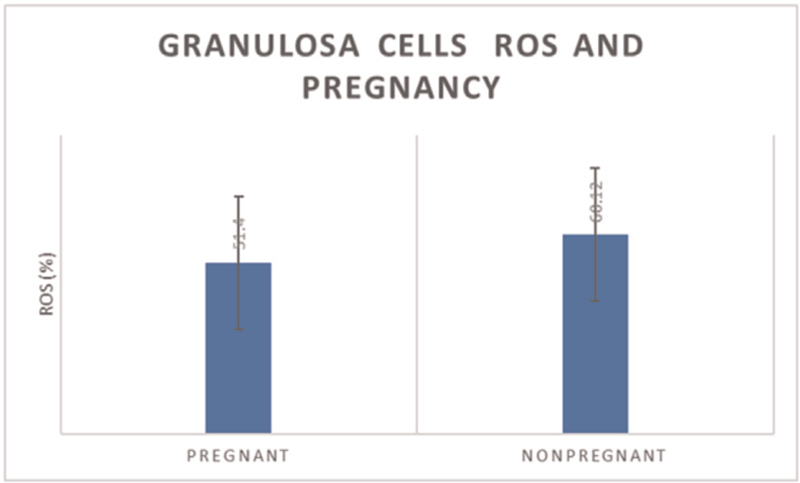
- Granulosa cells ROS and pregnancy outcomes. ROS, reactive oxygen species. t-statistic value = –1.408; DF = 46; P = 0.1658.
The P value indicates that ROS measured in granulosa cells had no significant impact on the pregnancy outcomes.
THE MDA Conc. was calculated using the standard graph and values were estimated [Figure 1 and Table 1]. The MDA concentration in the FF of pregnant women was found to be 4.32 ± 2.35 as compared to 6.81 ± 2.92 in nonpregnant women. This difference was statistically highly significant with P < 0.0001 [Figure 3].
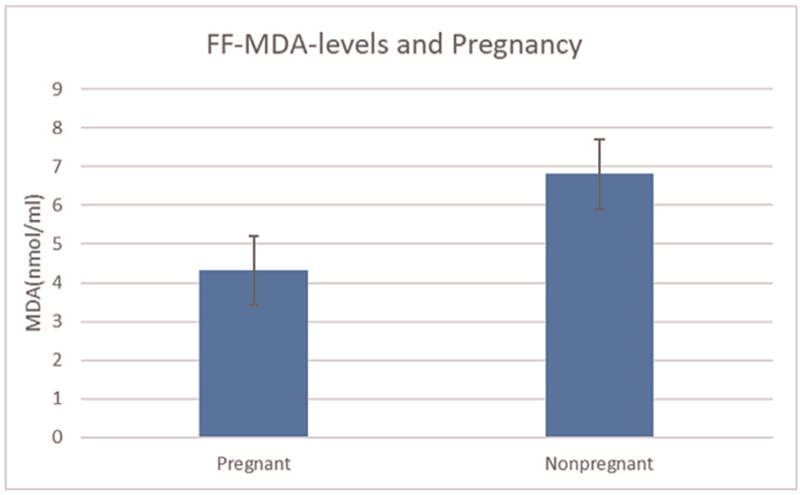
- MDA concentration of follicular fluid and pregnancy outcomes. MDA, malondialdehyde. t-statistic value = 5.132; DF = 46; P < 0.0001; highly significant.
Further, granulosa ROS and FF-MDA levels were analyzed with respect to the number of metaphase II (MII) oocytes and grade A embryos. Figure 4 shows that granulosa ROS levels had no effect on the number of mature oocytes obtained.

- Granulosa cells ROS and MII oocytes. The value of correlation coefficient r = 0.08924415. MII, metaphase II; ROS, reactive oxygen species.
Figure 5 shows FF-MDA levels and the number of MII oocytes obtained. The value of correlation coefficient r = 0.1672337 indicates that ROS in FF did not have any effect on the number of MII oocytes.
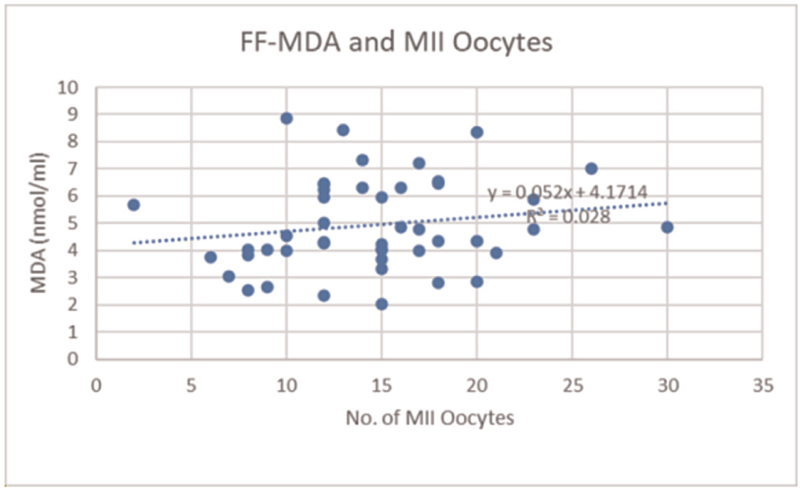
- FF-MDA levels and the number of MII oocytes. FF, follicular fluid; MDA, malondialdehyde; MII, metaphase II
The granulosa cell ROS levels showed negative correlation with good quality grade A embryos as shown in Figure 6. The correlation coefficient r was −0.441627604.
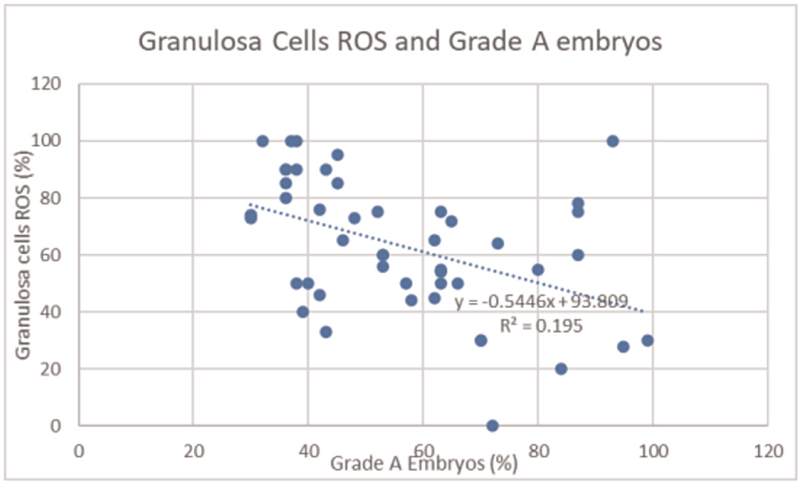
- Granulosa cells ROS and grade A embryos. ROS, reactive oxygen species
Again, a negative correlation was seen between FF-MDA and good quality embryos [Figure 7]. The value of correlation coefficient r was −0.048413367.
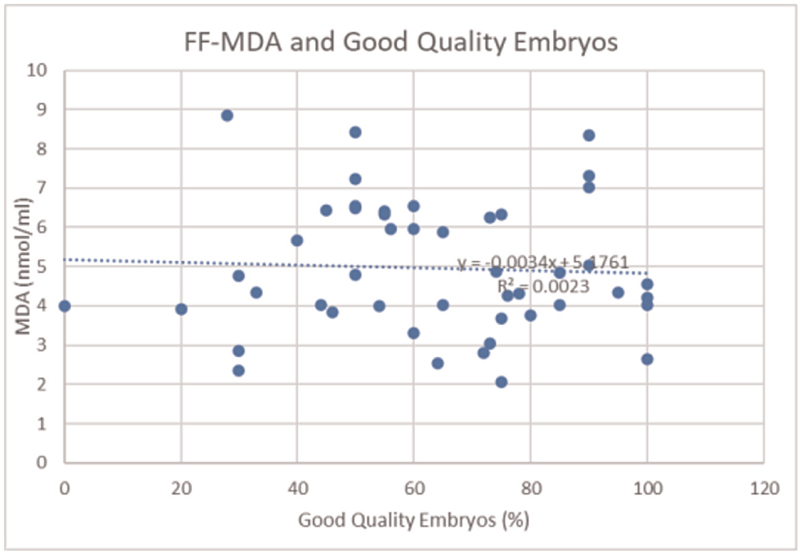
- FF-MDA and good quality embryos. FF, follicular fluid; MDA, malondialdehyde
DISCUSSION
In the present study, we analyzed the levels of ROS in the granulosa cells by flow cytometry and lipid peroxidation levels and products of protein oxidation (MDA concentrations) in the FF of women undergoing IVF. Our study was to measure the impact of OS parameters on embryo quality, number of mature oocytes, and also the pregnancy outcome.
Granulosa cells confirm that successful maturation and developmental competency of oocytes take place by providing important nutrients and maturation-enabling factors. The cells also protect the oocytes from OS injury with help of their own antioxidants during the process of maturation of oocytes.[8] Granulosa cells are very sensitive to ROS and this plays an important role in its apoptosis induction pathway[9] specific to endogenous H2O2. During the same time, when ROS is at high level, it can also cause cell dysfunction and cell death. It has also been proved that reduced glutathione depletion sensitizes in the granulosa cells to toxicant and induces apoptosis.[10] Our results showed that granulosa cell ROS production had significant negative correlation with grade A embryos. The ROS values of the granulosa cells were not significant in our study as they did not correlate with the pregnancy result. This may be due to the limitation of our study because the ROS estimate of the granulosa cells was not performed on the same day.
FF has many low molecular weight metabolites that directly or indirectly regulate OS.[11] It can be seen that increased ROS activity in FF is lethal to embryo formation and it also affects the cleavage rate and the amount of fragmentation.[12] Hence, FF provides a metabolically active environment in which oocytes live along with steroids, hormones, growth factors, cytokines, granulosa cells, macrophages, and leukocytes.[4]
Increased ROS activity in FF may be toxic to embryo formation,[13] but a physiologic amount may be a representation of healthy developing oocytes. Becatti et al. have recently shown in their study that infertile women undergoing IVF have significantly altered redox status reflected by FF redox status. The data clearly states that ROS plays a role in infertility and treatment outcome [Tables 1 and 2]. It affects the quality of embryos and also the pregnancy outcome (P < 0.0001). OS causes damage in all cells of mammals. There are studies that support the fact that a high level of ROS has a negative impact on female infertility.

Agarwal et al.[14] in their study attempted to determine the relationship between various female infertility problems and ROS levels in peritoneal fluids and concluded that there were significant differences in control group ROS levels and idiopathic infertility.
Although there are some studies that show that ROS does not affect the embryo quality, for example, in a study by Charalampos Siristatidis et al, it was observed that there was no correlation between ROS and embryo quality. ROS levels were measured in FF from follicular aspirates using enzyme-linked immunosorbent assay and through Poisson model they came to a conclusion that ROS had no role in embryo quality (P > 0.05).[15]
Another study observed that there was a significant relationship between ROS levels in FF and oocyte maturation and pregnancy outcome.
The study was done by measuring ROS and total antioxidant capacity (TAC) present in FF with the help of chemiluminescence. The result stated that patients who suffered from endometriosis had a higher level of ROS (1.44 ± 0.23) than those who did not suffer from endometriosis (0.60 ± 0.17).[16] A study of Oyawoye et al. evaluated baseline total antioxidant capacity TAC from follicular aspirates using ferric reducing antioxidant power and they came up to a conclusion that baseline TAC had no difference in FF whether the follicle had an oocyte or not but was significantly higher in fluids from follicles where the embryo survived and was transferred.[5] The pathological level of ROS affects embryo quality that results in blocking of embryonic development. Sajal Gupta et al. in their study showed the impact of ROS on embryo quality. They stated that high oxygen tension activates various oxidase enzyme systems in the cells that generate free radicals. Lower tension produced good quality embryos.[17] This study goes in agreement with our results that show that high ROS levels in FF or granulosa cells impair embryo quality.
CONCLUSION
OS is a real threat to embryos especially in vitro and they have a negative impact on the IVF outcomes. To reduce ROS levels, gametes and embryos should be handled as little as possible and exposed to low concentration of oxygen and visible lights.
We conclude that the ROS concentration in FF can be used as a marker to record the results of the pregnancy results. High ROS values in FF reduce the quality of the embryos and have a negative impact on the pregnancy rate. MDA levels or lipid peroxidation assay can also be used for predicting the outcome of IVF. Granulosa cell ROS can also be used as a marker for OS if done immediately after collection. The assessment of redox state of both granulosa cells and FF can be used as an effective marker of OS in assisted reproductive technology (ART) patients.
Prophylactic oral antioxidant treatment as well as culture media supplement with antioxidants can also help in reducing OS. OS in relation to female reproductive system can help us in getting better pregnancy outcomes. The results found that all identifiable modifiable factors that can help reduce OS in FF can be noninvasive therapy to reduce infertility.
Ethical and financial considerations
The ethical clearence has been taken for the study by independent ethics committee of indian fertility society.
There has been no financial implications in the study whatsoever.
Financial support and sponsorship
RIDGE IVF Pvt. Ltd.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Reactive oxygen species level in follicular fluid–embryo quality marker in IVF? Hum Reprod. 2006;21:2403-7.
- [Google Scholar]
- Prevention of twin pregnancy after in vitro fertilization or intracytoplasmic sperm injection based on strict embyo criteria: a prospective randomized clinical trial. Human Reprod. 1999;14:2581-7.
- [Google Scholar]
- Role of oxidative stress and antioxidants in andrology and assisted reproductive technology. J Androl. 2004;25:5-18.
- [Google Scholar]
- Role of oxygen species in the pathophysiology of humanreproduction. Fertil Steril. 2003;79:829-43.
- [Google Scholar]
- Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum Reprod. 2003;18:2270-4.
- [Google Scholar]
- Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81:973-6.
- [Google Scholar]
- Upper control limit of reactive oxygen species in follicular fluid beyond which viable embryo formation is not favorable. Reprod Toxicol. 2010;29:447-51.
- [Google Scholar]
- “Oxidative stress may be a piece in the endometriosis puzzle. Fertil Steril. 2003;79:1288-93.
- [Google Scholar]
- Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012;86:27.
- [Google Scholar]
- Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44:280-7.
- [Google Scholar]
- Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 1998;13:998-1002.
- [Google Scholar]
- A biochemical approach to detect oxidative stress in infertile women undergoing assisted reproductive technology procedures. Int J Mol Sci. 2018;19:592.
- [Google Scholar]
- The effect of reactive oxygen species on embryo quality in IVF. In Vivo. 2016;30:149-53.
- [Google Scholar]
- The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Wom Med. 2000;45:314-20.
- [Google Scholar]
- The impact of reactive oxygen species on early human embryos systemic review of literature. Embryo Talk. 2006;1:87-98.
- [Google Scholar]







