Translate this page into:
Correlation Between Luteal Phase Serum Progesterone Levels and Pregnancy Outcome in Frozen Embryo Transfer Cycles: A Prospective Cohort Study

*Corresponding author: Dr. Manika Sachdeva, MS, DNB, Diploma in Clinical ART, Department of Reproductive Medicine, Akanksha IVF Centre, Room No. 628, Mata Chanan Devi Hospital, Janak Puri, New Delhi 110058, India manikasachdevajoshi@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sachdeva M, Nayar KD, Singh S, Sethi A, Kant G, Arora S, et al. Correlation Between Luteal Phase Serum Progesterone Levels and Pregnancy Outcome in Frozen Embryo Transfer Cycles: A Prospective Cohort Study. Fertil Sci Res. 2024;11:11. doi: 10.25259/FSR-11-11
Abstract
Objectives
To study the correlation between luteal phase serum progesterone (P4) levels and pregnancy outcome in frozen embryo transfer (FET) cycles and to find out the cut-off level of luteal phase serum P4 which favours successful pregnancy outcome in FET cycles.
Material and Methods
This prospective cohort study included 100 women undergoing hormone replacement therapy (HRT)-FET cycle at Akanksha IVF Centre from December 2023 to June 2024, fulfilling the inclusion and exclusion criteria. Serum P4 levels were measured for all the patients in the luteal phase (days 21–23) after FET with intramuscular P4 as luteal phase support. Pregnancy outcome was assessed in terms of implantation rate, biochemical pregnancy rate, clinical pregnancy rate (CPR), first-trimester miscarriage rate and ongoing pregnancy rate.
Results
The most favourable pregnancy outcomes were observed at serum P4 levels 25.1-35 ng/mL. A statistically significant association was seen between the luteal phase serum P4 levels and implantation rate (36.08%), biochemical pregnancy rate (4%), CPR (52%) and ongoing pregnancy rate (42%). According to the receiver operating characteristic (ROC) curve analysis, the optimal cut-off value for favourable outcomes was determined to be 22.3 ng/mL. However, the analysis also indicated that the luteal phase serum P4 levels did not reliably predict clinical or ongoing pregnancy.
Conclusion
Larger studies are needed to establish a threshold level of serum P4 in the luteal phase that can differentiate between successful and unsuccessful implantation. However, it is still uncertain whether the issue of unsuccessful implantation can be resolved once it is detected on days 21–23 of the HRT-FET cycle.
Keywords
Luteal phase
progesterone
frozen embryo transfer
cryopreserved embryo transfer
pregnancy outcome
INTRODUCTION
The progesterone (P4) secretion by the corpus luteum following ovulation plays a vital role in orchestrating the necessary changes in the endometrium for successful implantation and a positive pregnancy outcome. P4 has a regulatory effect on key ultrastructural features of the secretory phase endometrium, such as giant mitochondria, glycogen accumulations in subnuclear regions, pinopodes and the nucleolar channel system, all of which facilitate the process of implantation.[1] Additionally, it is believed to support implantation by stimulating the immune system to generate non-inflammatory T-helper-2 cytokines.[2,3]
In the presence of optimum levels of P4, CD56 cells are known to produce a substance known as P4-induced blocking factor (PIBF), which is believed to possess anti-abortive properties.[4] Furthermore, P4 plays an important role in enhancing nitric oxide production, which, in turn, promotes improved blood circulation and oxygen supply to the endometrium.[5]
Luteal phase defect (LPD) is commonly observed in most of the assisted reproductive technology (ART) cycles, which often necessitates the supplementation of additional P4. The causes of LPD have been associated with various factors, such as the removal of granulosa cells during oocyte retrieval, extended pituitary suppression due to agonist administration in gonadotropin-releasing hormone (GnRH) agonist cycles or elevated levels of serum estradiol and P4 that inhibit endogenous luteinising hormone release via negative feedback mechanisms at the hypothalamic-pituitary-ovarian axis.[6–8] As a result, there is a consensus within the medical community regarding the requirement for P4 supplementation during the luteal phase of all stimulated ART cycles.[9,10]
The significance of P4 in the context of implantation and early pregnancy is undeniable, and it’s reasonable to consider that LPD could potentially contribute to unsuccessful implantation and early pregnancy loss (EPL) during ART cycles.[11,12] Research indicates that in fresh in vitro fertilisation-embryo transfer (IVF-ET) cycles, where conceptions occur, there is a more rapid increase in P4 levels and higher luteal phase P4 levels compared to cycles without conception.[13]
However, achieving optimal pregnancy rates in frozen embryo transfer (FET) cycles hinges on adequate luteal phase support (LPS), primarily through exogenous P4 administration.[14] Literature is scarce regarding luteal phase serum P4 levels in FET cycles, in which there is no endogenous production of P4 due to the absence of ovulation and, in turn, the corpus luteum.[15]
Endogenous production of P4 during pregnancy may begin around week 6, coinciding with the uteroplacental shift.[16] Therefore, in artificial cycles, serum P4 levels during the mid-to-late luteal phase transition might solely rely on the dosage of exogenous P4 administered to the patient.[17]
A specific threshold for luteal phase serum P4 that may affect the outcomes of hormone replacement therapy (HRT)-FET cycles has also not been established till now. Thus, we conducted a study to find a correlation between luteal phase serum P4 levels and pregnancy outcome in FET cycles and the cut-off level of luteal phase serum P4 which favours successful pregnancy outcome.
MATERIAL AND METHODS
Ethics
Ethical clearance was given by the Independent Ethical Committee, Indian Fertility Society via an Approval Letter F.1/IEC/IFS/2023/No.14 dated 20.12.2023.
Study design
This prospective cohort study included 100 women undergoing the HRT-FET cycle at Akanksha IVF Centre from December 2023 to June 2024, fulfilling the inclusion and exclusion criteria.
Inclusion criteria
Patients diagnosed with either primary or secondary infertility, aged 21–40 years, having a BMI (body mass index) of <30 kg/m2 with a normal endocrinological profile and normal uterus on 2D (two-dimensional) ultrasonography, undergoing HRT-FET of a good quality embryo (grade A cleavage stage embryo or blastocyst) at our centre were included in this study.
Exclusion criteria
Patients with a history of recurrent implantation failure, having any uterine defects like Asherman’s Syndrome or any space-occupying lesions in the uterus like fibroids or adenomyosis were excluded from the study.
Methodology
Endometrial preparation for HRT-FET was done by sequential incremental administration of oral estradiol valerate (Tab Progynova® 2 mg, Bayer Zydus Pharma Pvt Ltd, India) + transdermal 17β estradiol gel (Estogel, Intas Pharmaceuticals Ltd, India) until endometrial thickness was ≥8 mm, followed by estradiol combined with injectable intramuscular P4 (Injection Uterone 100 mg, Jagsonpal Pharmaceuticals Ltd, India) as LPS. FET was done on P+3/P+5 per day, depending upon the stage of the embryo that was transferred. Serum P4 was measured 3 days after blastocyst transfer or 5 days after cleavage stage transfer, corresponding to the luteal phase of the cycle (days 21–23). The serum P4 test was conducted for all patients at the same laboratory using electrochemiluminescence immunoassay (ECLIA) to ensure consistent results. The test was performed in the morning, at least 10–12 hours after the last injection. Urine pregnancy test or serum Beta-human chorionic gonadotropin (hCG) levels were checked 14 days after FET. In case of a positive pregnancy test, patients were followed up at 6–7 weeks period of gestation to assess the pregnancy outcome of that FET cycle. In case of a viable pregnancy, patients were followed up at 11–12 weeks period of gestation with an ultrasonography report to confirm foetal well-being (ongoing pregnancy) and to see how many underwent first-trimester miscarriage.
Primary outcome
Correlation between luteal phase serum P4 levels and pregnancy outcome in FET cycles.
Cut-off level of luteal phase serum P4, which favours successful pregnancy outcome.
Secondary outcome
Implantation rate
Biochemical pregnancy rate
Clinical pregnancy rate
First-trimester miscarriage rate
Ongoing pregnancy rate
Statistical analysis
Categorical variables were presented in number and percentage (%), and continuous variables were presented as mean ± SD (standard deviation) and median. The normality of data was tested by the Shapiro-Wilk test. If the normality was rejected, then the non-parametric test was used. Statistical tests were applied as follows:
Quantitative variables were associated using an unpaired t-test/Mann-Whitney Test (when the data sets were not normally distributed) with the outcome.
Qualitative variables were associated using the chi-square test/Fisher’s exact test.
The receiver operating characteristic (ROC) curve was used to assess the cut-off point, sensitivity, specificity, positive predictive value and negative predictive value of the luteal phase serum P4 for predicting successful pregnancy outcome.
A p-value of less than 0.05 was considered significant. The data was entered into an MS Excel spreadsheet, and the analysis was done using the Statistical Package for Social Sciences (SPSS) version 25.0.
RESULTS
A total of 100 patients undergoing HRT-FET were included in our study. The mean age of our patients was 33.58 years, and the mean BMI was 23.91 kg/m2. Patients were divided into four groups on the basis of raw data of the luteal phase serum P4 levels available after completion of the study: Group 1 (<15 ng/mL), Group 2 (15.1-25 ng/mL), Group 3 (25.1-35 ng/mL) and Group 4 (>35 ng/mL). The baseline characteristics of all four groups were similar [Table 1].
| P4 groups (ng/ml) (Mean ± SD) | P4 <15 | P4 15.1–25 | P4 25.1–35 | P4 <35 | p-value |
| Age (years) | 34 ± 4 | 33.26 ± 3.34 | 34.37 ± 3.83 | 32.32 ± 3.46 | 0.158 |
| BMI (kg/m2) | 24.38 ± 1.64 | 23.07 ± 2.4 | 24.08 ± 2.75 | 24.42 ± 2.78 | 0.436 |
| Duration of infertility (years) | 8.4 ± 7.38 | 6.07 ± 3.55 | 7.56 ± 3.14 | 5.36 ± 3.55 | 0.386 |
| AMH (ng/ml) | 4.2 ± 3.87 | 3.63 ± 3.37 | 3.6 ± 5.02 | 3.72 ± 3.22 | 0.3 |
| Antral follicle count | 12.6 ± 10.19 | 12.15 ± 9.08 | 9.95 ± 7.21 | 10.36 ± 4.28 | 0.117 |
| Endometrial thickness (mm) | 9.84 ± 0.58 | 9.65 ± 0.84 | 9.29 ± 0.89 | 9.22 ± 0.94 | 0.102 |
SD = Standard deviation, P4 = Progesterone, BMI = Body Mass Index, AMH = Anti Mullerian Hormone
Out of the 56% of patients who became pregnant, 52% were clinical pregnancies, and 4% were biochemical pregnancies [Figure 1]. The overall implantation rate among our study population was 36.08%. A significant association was found between luteal phase serum P4 levels and implantation rate, with the highest implantation rate (45.45%) seen in the 25.1–35 ng/mL group (p = 0.004). The clinical pregnancy rate (CPR) among our study population was 52%, with the highest CPR in the third group (25.1-35 ng/mL; 68.3%). A significant association was seen between CPR and luteal phase serum P4 levels (p = 0.018). A significant association was also found between the biochemical pregnancy rate (4%) and luteal phase serum P4 levels (p = 0.013), with all the biochemical pregnancies belonging to the second P4 group (15.1-25 ng/mL). No significant association was seen between miscarriage rate (10%) and luteal phase serum P4 levels (p = 0.542). A significant association was found between the ongoing pregnancy rate (42%) and luteal phase serum P4 levels amongst our study population (p = 0.010), with the highest ongoing pregnancy rate in the third group (25.1-35 ng/mL) followed by the second group (15.1-25 ng/mL) [Figure 2]. The CPR and ongoing pregnancy rate peaked around the 25 ng/mL mark [Figure 3]. Both below and above this value, the pregnancy rates showed a decreasing trend.
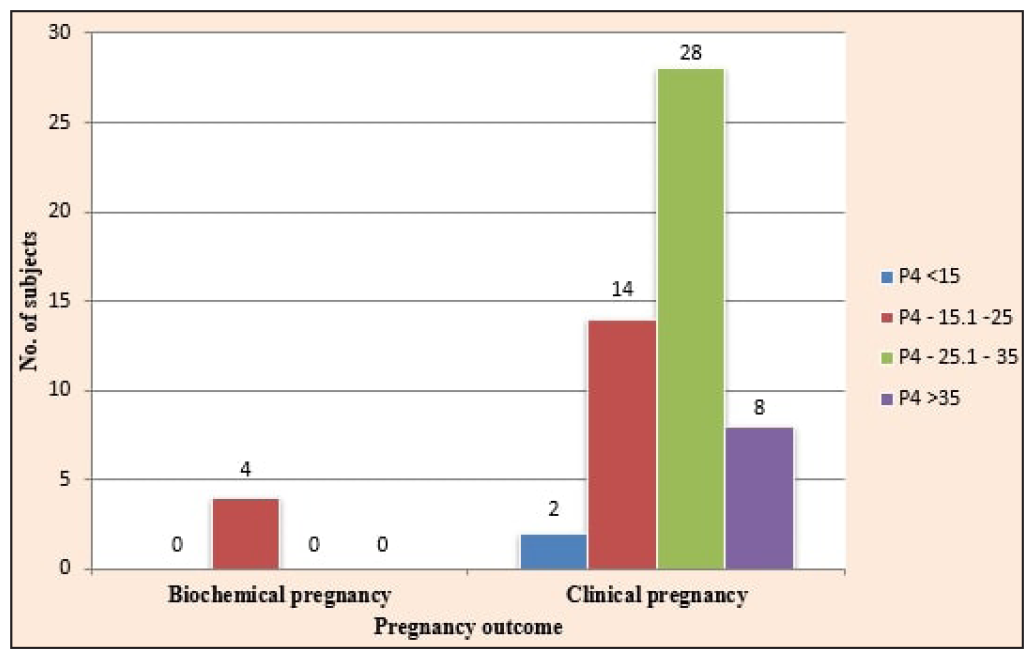
- Distribution of study subjects according to pregnancy outcome at 6–7 weeks period of gestation.
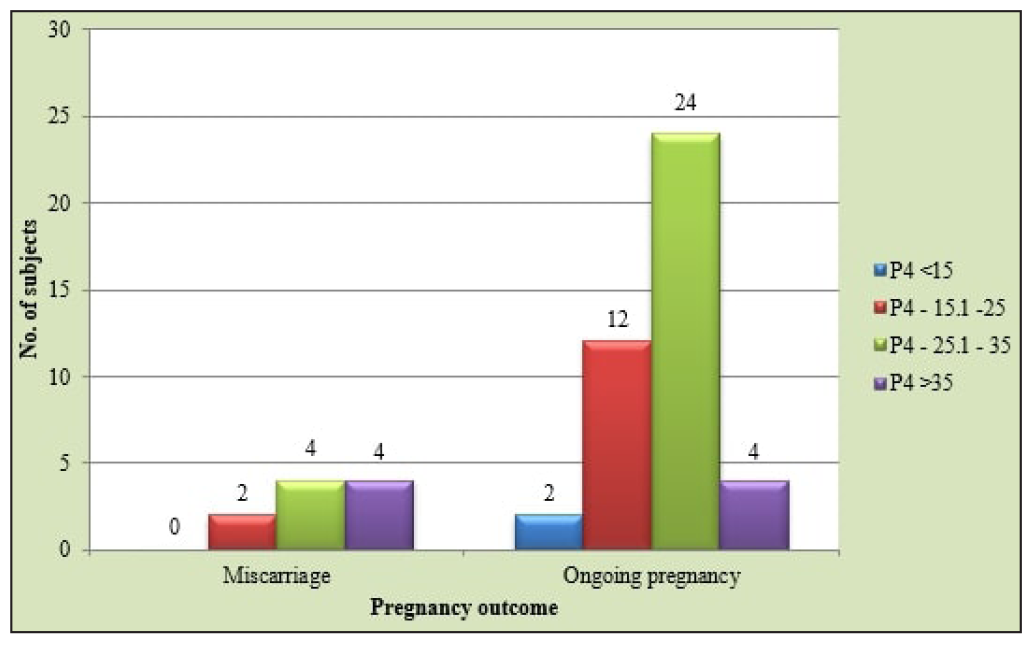
- Distribution of study subjects according to pregnancy outcome at 12 weeks period of gestation.
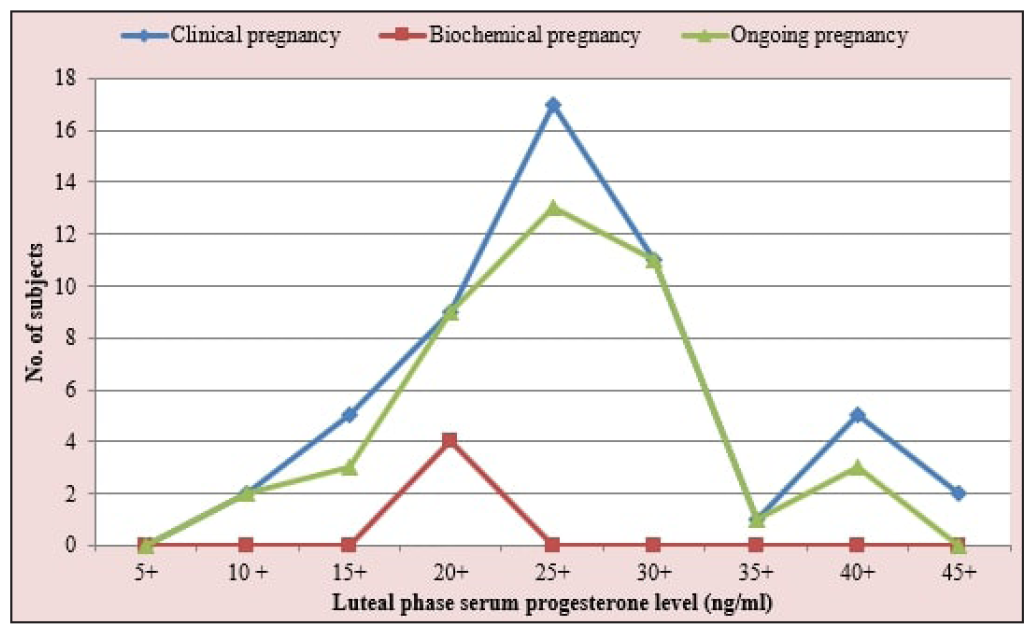
- Pregnancy outcome in relation to the luteal phase serum progesterone levels (n = 100).
The ROC curve defined an optimal cut-off value for serum P4 of 22.3 ng/mL, with a sensitivity of 86.5% and specificity of 33.3% for predicting clinical pregnancy [Figure 4] and a sensitivity of 88.1% and specificity of 31% for predicting ongoing pregnancy [Figure 5]. The ROC analysis indicated that the luteal phase serum P4 levels were neither predictive of clinical pregnancy, being the area under the curve (AUC) (95% CI [confidence interval]) = 0.543 (p-value = 0.456), nor predictive of ongoing pregnancy with AUC (95% CI) = 0.497 (p-value = 0.955).
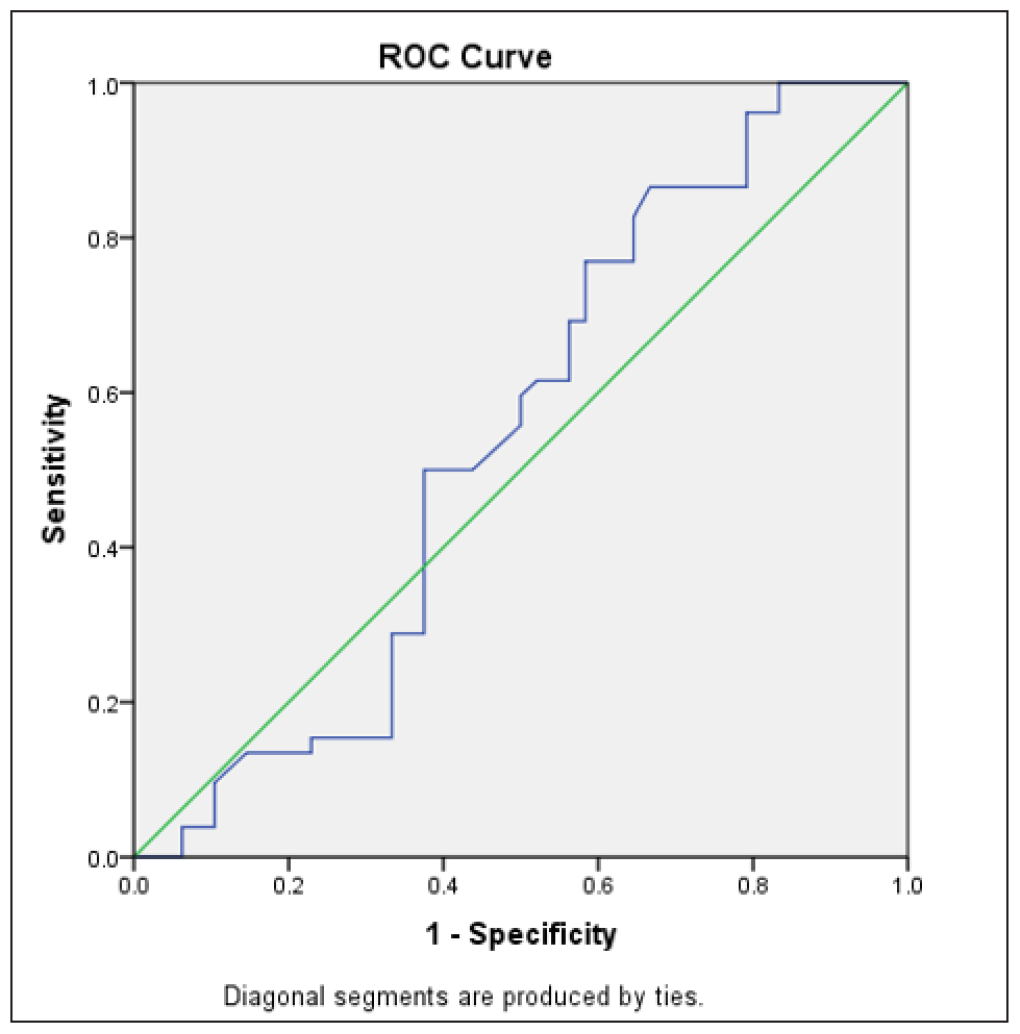
- Receiver Operating Characteristic (ROC) curve for predicting clinical pregnancy (n = 100).
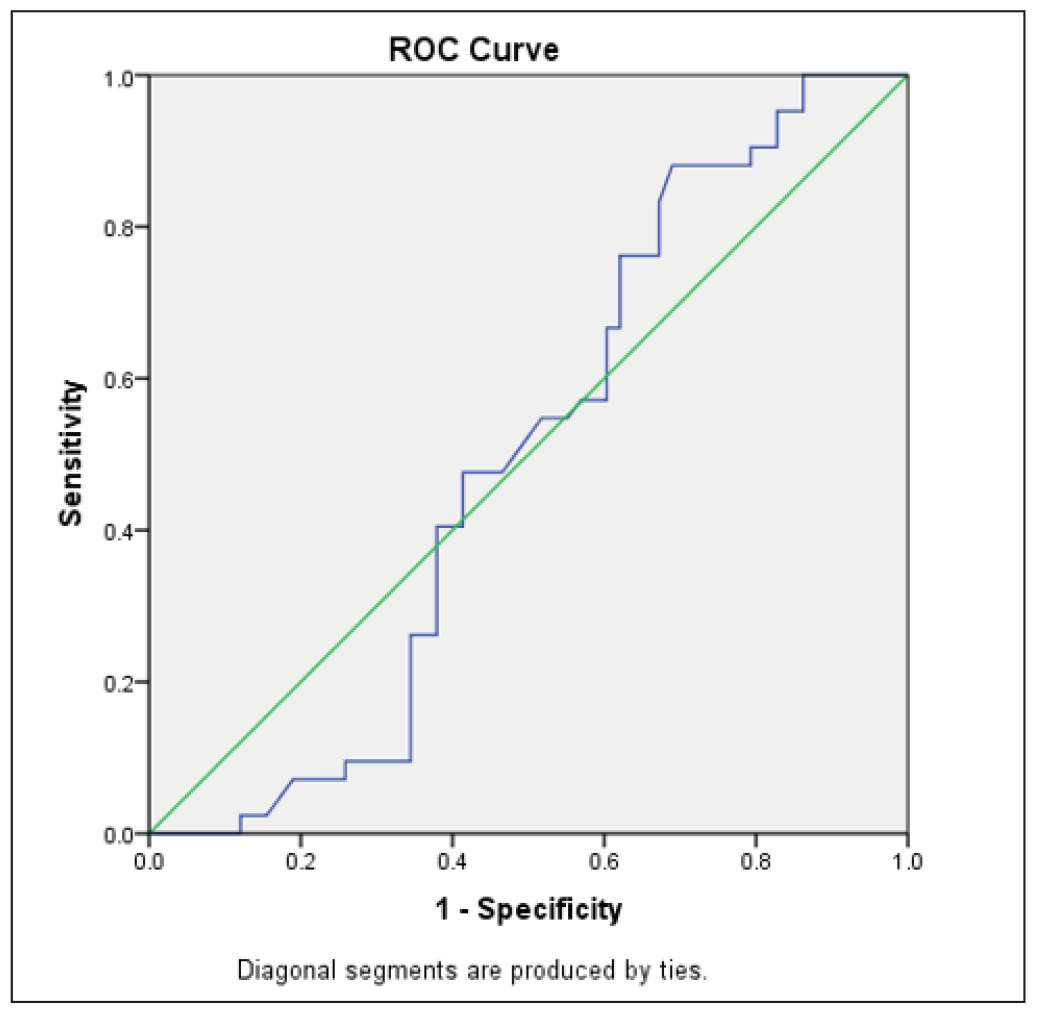
- Receiver Operating Characteristic (ROC) curve for predicting ongoing pregnancy (n = 100).
DISCUSSION
Out of the 194 embryos transferred in 100 patients undergoing HRT-FET, 70 embryos were implanted, resulting in an overall implantation rate of 36.08%. A significant association was found between the luteal phase serum P4 levels and implantation rate, with the highest implantation rate (45.45%) seen in the 25.1 to 35 ng/mL group. Yovich et al. identified that an ideal P4 range of 70–99 nmol/L (28–39.6 ng/mL; p < 0.005) was linked to optimal outcomes in their study population. P4 levels below 50 nmol/L (20 ng/mL) or above 99 nmol/L (39.6 ng/mL) were found to correlate with reduced implantation rates.[17]
Among our study population, the CPR was significantly higher in the third group (25.1-35 ng/mL; 68.3%), followed by the second group (15.1-25 ng/mL; 51.9%; p = 0.018). In the study by Yovich et al., the CPR among 529 HRT-FET cycles was found to be significantly high in the mid-luteal serum P4 group 70–99 nmol/L (28–39.6 ng/mL).[17] Similarly, in a study conducted by Varma et al. on HRT-FET cycles with intramuscular P4 as LPS, the CPR was found to be most significant in the P4 group 32.2–43.7 ng/mL (p = 0.02).[18]
The biochemical pregnancy rate was also observed to have a significant association with the luteal phase serum P4 levels (p = 0.013), with the highest biochemical pregnancy rate found in the second P4 group (15.1–25 ng/mL; 14.8%). Kaur et al. found a significantly higher biochemical pregnancy rate in the FET group with P4 levels <15 ng/mL (p = 0.024). This implies that an adequate level of serum P4 is essential to ensure successful implantation of the embryo and pregnancy maintenance. An embryonic factor may play a role, though all patients in our study were under 40 years old, suggesting a comparable rate of aneuploidy among them.[15]
Overall, 10% of patients in our study population suffered a first-trimester miscarriage. The highest miscarriage rate was seen in the fourth P4 group (>35 ng/mL; 18.2%), but it was not statistically significant. Lek et al. found a cut-off level of 35 nmol/L (14 ng/mL) as a predictor of miscarriage, which was not comparable with our study. Thus, we can infer that an optimum level of serum P4 is required for pregnancy sustenance; both below and above this level, the risk of miscarriage increases.[19]
Humaidan et al. found an inverse correlation between the mid-luteal serum P4 concentrations and the rate of biochemical pregnancies. He also observed a significant reduction in the EPL rate, from approximately 80% to 10%, as mid-luteal-phase P4 levels rose from around 40 nmol/L (12.57 ng/mL) to approximately 80–100 nmol/L (25.15-31.44 ng/mL).[20]
Alsbjerg et al. investigated the reproductive outcomes in patients undergoing frozen-thawed ET before and after increasing the dosage of vaginal P4 gel from 90 mg to 180 mg. They reported a notable decrease in the EPL rate (67% versus 44%; p = 0.014) and a significant increase in the delivery rate (9% versus 21%; p = 0.002) with higher P4 supplementation.[21]
A significant association was found between the ongoing pregnancy rate (42%) and luteal phase serum P4 levels in our study (p = 0.010), with the highest ongoing pregnancy rate in the P4 range 25.1-35 ng/mL (58.6%). Labarta et al. found an overall ongoing pregnancy rate of 47.2% in their study, which is similar to the result of our study (42%).[22] Most previous studies on mid-luteal serum P4 levels in FET cycles followed the patients till delivery and hence calculated the live birth rate.[18] Due to a paucity of time owing to a relatively short study period, we could not follow the patients till childbirth.
The ROC curve defined an optimal cut-off value of 22.3 ng/mL luteal phase serum P4 level among our study subjects for a favourable pregnancy outcome. Labarta et al. found a cut-off of 9.95 ng/mL in the mid-luteal phase, which is not at all comparable to our cut-off value. This can be explained by the fact that Labarta et al. used vaginal P4 400 mg twice daily as the LPS, whereas we used intramuscular P4 100 mg/day, which increases the serum P4 levels much more profoundly as compared to vaginal P4 which has a more significant first uterine pass effect.[22]
Ellenbogen et al. concluded in their study that a mid-luteal P4 level of >15 ng/mL increased the odds of getting pregnant by 3.2 times.[23] Kaur et al. also observed that in Indian women, mid-luteal serum P4 levels <15 ng/mL negatively impact pregnancy outcomes in both fresh and frozen-thawed ET cycles.[15] Mitwally et al. also found a similar cut-off level of 15 ng/mL.[24]
On the contrary, Aslih et al. conducted a prospective randomised controlled trial, revealing that biochemical pregnancy, clinical pregnancy and live birth rates showed no significant differences between groups, regardless of P4 levels on day 7 of the luteal phase (P4 <15 or >15 ng/mL). They found that even increasing P4 dosage in the low P4 group did not alter these outcomes, suggesting that enhancing P4 levels late in the luteal phase may not sufficiently improve the endometrial environment.[25]
Strengths
There are only a few studies worldwide that have demonstrated the association between the luteal phase serum P4 levels and pregnancy outcomes when intramuscular P4 is used as LPS in HRT-FET cycles. Most of the studies have used vaginal P4 as LPS and include only fresh ET cycles.
Limitations
Single-centre study with a small sample size.
Shorter duration of study.
Patients could not be followed till childbirth due to paucity of time owing to a shorter study period.
CONCLUSION
Larger studies are needed to establish a threshold level of serum P4 in the late luteal phase that can differentiate between successful and unsuccessful implantation. However, it is still uncertain whether the issue of unsuccessful implantation can be resolved once it is detected on days 21–23 of the HRT-FET cycle. It is also unclear whether the variation in serum P4 levels among patients, despite receiving identical P4 doses for LPS, is a contributing factor or merely a reflection of pregnancy outcomes. We recommend all institutes to identify their personalised ideal range of luteal phase serum P4 levels according to the HRT-FET protocol followed in their centre, the method of testing used and the timing of the test. Individualisation of the LPS based on that range can then be done to improve pregnancy outcomes.
Ethical approval
The research/study was approved by the Institutional Review Board at the Independent Ethical Committee - Indian Fertility Society, approval number F.1/IEC/IFS/2023/No.14, dated 20-12-2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Premature Formation of Nucleolar Channel Systems Indicates Advanced Endometrial Maturation Following Controlled Ovarian Hyperstimulation. Hum Reprod. 2013;28:3292-300.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Progesterone and the Immunology of Pregnancy. J Steroid Biochem Mol Biol. 2005;97:389-96.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Progesterone and Progestin Therapy in Threatened Abortion and Preterm Labour. Front Biosci. 2008;13:1981-90.
- [CrossRef] [PubMed] [Google Scholar]
- The Progesterone Receptor Regulates Implantation, Decidualization, and Glandular Development via a Complex Paracrine Signaling Network. Mol Cell Endocrinol. 2012;357:108-18.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects of Dydrogesterone and Its Stable Metabolite, 20-alpha-dihydrodydrogesterone, On Nitric Oxide Synthesis in Human Endothelial Cells. Fertil Steril. 2006;86:1235-42.
- [CrossRef] [PubMed] [Google Scholar]
- The Luteal Phase After In-vitro Fertilization and Related Procedures. Hum Reprod. 1988;3:161-4.
- [CrossRef] [PubMed] [Google Scholar]
- An Update of Luteal Phase Support in Stimulated IVF Cycles. Hum Reprod Update. 2007;13:581-90.
- [CrossRef] [PubMed] [Google Scholar]
- Luteal Phase Support in In vitro Fertilization. Semin Reprod Med. 2015;33:118-27.
- [CrossRef] [PubMed] [Google Scholar]
- Ovarian Feedback, Mechanism of Action and Possible Clinical Implications. Hum Reprod Update. 2006;12:557-71.
- [CrossRef] [PubMed] [Google Scholar]
- The Pattern of Luteal Phase Plasma Progesterone and Estradiol in Fertile Cycles. Am J Obstet Gynecol. 1982;143:808-13.
- [CrossRef] [PubMed] [Google Scholar]
- Mid-Luteal Progesterone Concentrations are Associated with Live Birth Rates During Ovulation Induction. Reprod Biomed Online. 2011;22:449-56.
- [CrossRef] [PubMed] [Google Scholar]
- Preimplantation Hormonal Differences Between the Conception and Non-Conception Menstrual Cycles of 32 Normal Women. Hum Reprod. 1997;12:2607-13.
- [CrossRef] [PubMed] [Google Scholar]
- Active Corpus Luteum Function at Pre-, Peri- and Postimplantation is Essential for a Viable Pregnancy. Early Pregnancy. 1995;1:281-7.
- [PubMed] [Google Scholar]
- Obstetric Outcomes After Fresh Versus Frozen-thawed Embryo Transfers: A Systematic Review and Meta-analysis. JBRA Assist Reprod. 2018;22:253-60.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Impact of Mid-luteal Serum Progesterone Levels on Pregnancy Outcome in Fresh and Frozen Embryo Transfer Cycles in women of Indian ethnicity. Onco Fertil J. 2018;1:30-5.
- [CrossRef] [Google Scholar]
- Endogenous Progesterone Levels Could Predict Reproductive Outcome in Frozen Embryo Replacement Cycles Supplemented with Synthetic Progestogens: A Retrospective Cohort Study. Reprod Med Biol. 2018;18:91-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mid-luteal Serum Progesterone Concentrations Govern Implantation Rates for Cryopreserved Embryo Transfers Conducted Under Hormone Replacement. Reprod Biomed Online. 2015;31:180-91.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship of Midluteal Serum Progesterone Levels and Pregnancy Outcomes after day-3 Embryo-transfer (ET) in Hormonal Replacement Therapy (HRT) Cycles with Intramuscular Progesterone: A Prospective Cohort Study, Human Reproduction. . 2023;38(Supplement_1):dead093.843.
- [Google Scholar]
- Validation of Serum Progesterone <35nmol/L as a Predictor of Miscarriage Among Women with Threatened Miscarriage. BMC Pregnancy Childbirth. 2017;17:78.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rescue of Corpus Luteum Function with Peri-ovulatory HCG Supplementation in IVF/ICSI GnRH Antagonist Cycles in Which Ovulation was Triggered with a GnRH Agonist: A Pilot Study. Reprod Biomed Online. 2006;13:173-8.
- [CrossRef] [PubMed] [Google Scholar]
- Increasing Vaginal Progesterone Gel Supplementation After Frozen-Thawed Embryo Transfer Significantly Increases the Delivery Rate. Reprod Biomed Online. 2013;26:133-7.
- [CrossRef] [PubMed] [Google Scholar]
- Serum Progesterone Profile Across the Mid and Late Luteal Phase in Artificial Cycles Is Associated With Pregnancy Outcome. Front Endocrinol (Lausanne). 2021;12:665717.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mid-luteal Serum Progesterone and Estradiol Levels as Predictors of Pregnancy in IVF-ET Cycles: May Increasing the Dosage of Progesterone Supplementation Improve the Outcome? Fertil Steril. 2004;82:S205-6.
- [Google Scholar]
- Vaginal Micronized Progesterone Versus Intramuscular Progesterone for Luteal Support in Women Undergoing in Vitro Fertilization-embryo Transfer. Fertil Steril. 2010;93:554-69.
- [CrossRef] [PubMed] [Google Scholar]
- Can We Alter Pregnancy Outcome by Adjusting Progesterone Treatment at Mid-luteal Phase: A Randomized Controlled Trial. Gynecol Endocrinol. 2017;33:602-6.
- [CrossRef] [PubMed] [Google Scholar]








