Translate this page into:
Dynamics of HOXA10 expression in ectopic endometrium of a mouse model of endometriosis
Address for correspondence: Dr Deepak Modi, Molecular and Cellular Biology Laboratory, ICMR-National Institute for Research in Reproductive and Child Health, J.M. Street, Parel, Mumbai 400-012, India. E-mail: deepaknmodi@yahoo.com, modid@nirrch.res.in
-
Received: ,
Accepted: ,
How to cite this article: Mishra A, Modi D. Dynamics of HOXA10 expression in ectopic endometrium of a mouse model of endometriosis. Fertil Sci Res 2023;10:195-204.
Abstract
Introduction:
Homeobox gene A10 (HOXA10) is a transcription factor that plays a key role in maintaining endometrial homeostasis. In women with endometriosis, HOXA10 expression is downregulated, which is thought to cause progesterone resistance. However, it is unknown whether this downregulation is a cause or consequence of endometriosis.
Materials and Methods:
In this study, we used a mouse model of endometriosis and demonstrated that compared to the normal endometrium, the expression of HOXA10 is progressively downregulated during lesion development (from day 10 to day 65).
Results:
We observed that the expression of HOXA10 is lower in both well-differentiated and mixed types of endometriosis. During lesion development, the levels of HOXA10 were initially downregulated in epithelial cells more than in stromal cells. However, as the lesion development progressed further, the stromal expression was drastically reduced. While the nucleocytoplasmic ratio of HOXA10 was identical between control and endometriosis lesions at the initial stages, at later time points, HOXA10 remained largely nuclear, with little expression in the stroma.
Conclusion:
We conclude that the downregulation of HOXA10 is a consequence of endometriosis and may contribute toward its pathogenesis.
Keywords
Mouse
Endometrium
Epithelium
Stroma
Homeobox
Genes
INTRODUCTION
Endometriosis is a steroid hormone-dependent, chronic benign gynecological endometrial disorder affecting 10 to 15% of women of reproductive age and almost 190 million women globally.[1,2] Symptoms of endometriosis include chronic pelvic pain, dysmenorrhea, dyspareunia, and peritoneal inflammation. There are different theories related to the etiology of endometriosis. Among all the theories, Sampson's theory of retrograde menstruation is the oldest and the most widely accepted mechanism to explain endometriosis. Retrograde menstruation occurs in 70 to 90% of women; however, only 10% of women have endometriosis.[3] Thus, a modified theory of retrograde menstruation was proposed. According to this theory, when retrograde menstruation occurs, these cells become more invasive and migratory, and implant at an ectopic location, but other factors like hormones, genetic predisposition, inflammatory molecules, angiogenic molecules, and stem cells are also required for the growth and development of endometriosis.[4,5,6,7,8,9] Identities of such factors that contribute to pathogenies are not completely understood.
At the molecular level, there is an altered expression of many genes in women with endometriosis. Among these, homeobox gene A10 (HOXA10) is one of the key molecules reported to be altered in women suffering from endometriosis.[10] HOXA10 belongs to the homeobox gene A (HOXA) family and plays an important role in uterine biogenesis during embryonic development.[11] In the adult endometrium, HOXA10 acts as a transcription factor, and its signals need to be transmitted to the site of action to regulate the expression of target genes required for receptivity and decidualization for embryo implantation.[10,12,13,14,15] HOXA10 is characterized by a 183-bp highly conserved sequence that encodes a 61-amino acid region called the homeodomain, a deoxyribonucleic acid (DNA)-binding domain that recognizes a typical core DNA sequence of the promoter region (typically TAAT or TTAT of a target gene) and regulates expression of its target genes.[14,16]
In endometriosis, HOXA10 expression is altered in both eutopic and ectopic endometrium as compared to the endometrium of women without endometrial diseases.[17,18,19,20] Further, its expression is also altered in the ectopic endometrium as compared to the matched eutopic endometrium.[21,22,23,24,25] In addition, the levels of HOXA10 are lower in endometriotic lesions of baboons.[19] While the functional significance of the downregulation of HOXA10 in the pathogenesis of endometriosis, studies have shown that HOXA10 is required to regulate inflammatory markers and control endometrial epithelial cell proliferation and cell migration in other tissues.[13,26,27,28] As all these features are also noted in endometriosis, many of these features are likely mediated by the loss of HOXA10 to promote its pathogenesis.[29]
What causes the downregulation of HOXA10 is hitherto unknown. Some studies show that the HOXA10 gene consists of three fragments: F1 in the 5′ promoter region as well as F2 and F3, which are found within the intron between exons 1 and 2.[30] Hypermethylation of the 5′ promoter region is found to be associated with the altered expression of HOXA10 in both the eutopic and ectopic endometrium of women with endometriosis.[19,23,31,32,33] Other studies suggest that microribonucleic acid (microRNA) and single nucleotide polymorphisms in the coding region of HOXA10 are associated with altered expression of HOXA10 in women with endometriosis.[34,35] While there is adequate evidence to suggest that there is downregulation of HOXA10 in the ectopic endometrium of women with endometriosis, it is unknown if this reduction is a cause or consequence of endometriosis.
Understanding the regulation of HOXA10 in endometriosis is crucial to have an insight into the pathogenesis of endometriosis as we and others have earlier shown that HOXA10 is a key regulator of inflammatory pathways, cell cycle networks, Wnt/βcatenin pathways, and several other pathways which are also disrupted in women with endometriosis.[26,36,37] While work on human tissues provides critical insights into pathogenesis, it is neither ethical nor justifiable to conduct longitudinal studies in humans to understand disease progression. Furthermore, the results of human studies are confounded by differences in ethnicity, study design, patient selection criteria, stage of cycle, type of endometriosis, and previous interventions. Thus, fundamental studies are rather difficult to conduct in humans.
To address these issues, various animal models for endometriosis are developed that allow researchers to study lesion evolution and disease progression.[38,39] Among the various animal models to study endometriosis, the autotransplantation mouse model for endometriosis is well characterized and has demonstrated applicability in various areas of research. This model mimics various aspects of endometriosis including lesion evolution, steroid resistance,[40] estrogen synthesis,[41] and inflammation.[42]
Herein, we utilized this model to determine the spatial and temporal expression of HOXA10 in endometriosis tissues during lesion evolution.
Materials and methods
Animals and Animal Ethics
6-Month-old C57BL/6 strain mice were used for our study. This study was approved by the Institutional Animal Ethics Committee of the National Institute for Research in Reproductive and Child Health under project number 05/12.
Endometriosis Induction
Endometriosis was surgically induced in the mice as described previously.[42,43] In brief, mice were anesthetized and a small incision was made on the skin and muscle to expose the uterine horns. As shown in Figure 1a, the left uterine horn was removed, opened medially, and cut into small fragments. One fragment (0.8–1 mm) was sutured with the intestinal mesentery; muscle and skin were stitched back and mice were placed back in the cage. Mice were given postoperative care.
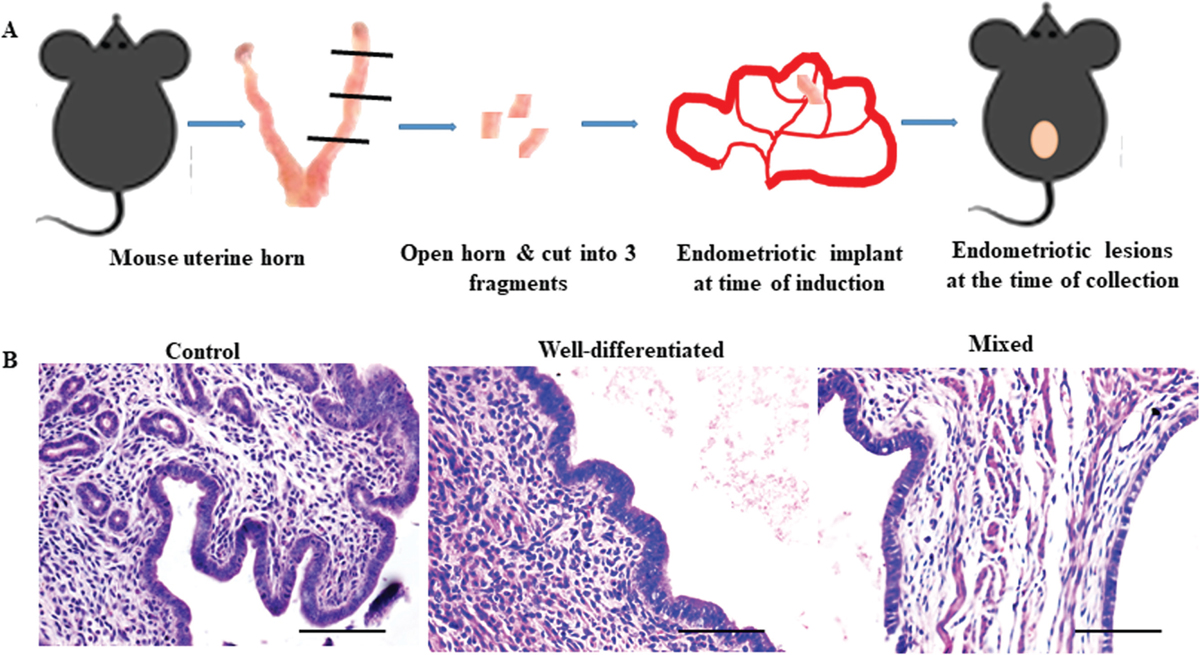
- Evaluation of endometriotic lesions in mice acquired during surgery. (a) Experimental strategy to induce endometriosis in mouse where a small uterine fragment was sutured to the same mouse's intestinal mesentery in order to cause endometriosis (b) Hematoxylin and eosin-stained eutopic endometrium of control mice and ectopic endometrium representing Well-differentiated, and mixed type of endometriosis similar to human (n = 21). Scale bar, 50 µm.
Experimental Tissue Collection
Animals in the diestrus phase were sacrificed on days 10, 15, 20, 30, 45, 60, and 65 after surgery. To extract RNA, tissue attached to intestinal mesentery was removed and stored in TRIzol reagent (Invitrogen, California, USA). For immunostaining, tissue was fixed in 4% Paraformaldehyde (Sigma Aldrich, Missouri, USA) for paraffin embedding and sectioning. For controls, uterus from C57BL/6 strain mice of the same age and phase were also collected and processed the same as above.
Real-Time Quantitative Polymerase Chain Reaction
As previously mentioned,[44,45] total RNA was extracted using TRIzol reagent (Invitrogen, Massachusetts, USA) and reverse transcribed using a high-capacity complementary DNA (cDNA) reverse transcriptase kit (Applied Biosystems, Massachusetts, USA). Relative messenger RNA (mRNA) levels of 18s rRNA and HOXA10 were measured by quantitative polymerase chain reaction (qPCR) employing iQ SYBR green chemistry (Bio-Rad, CA, USA). The Bio-Rad CFX-96 thermal cycler was used to perform PCR and every PCR reaction was run in duplicate, gene expression was adjusted to equal 18S levels, and fold change was determined using the previously mentioned Pfaffl technique (Table 1).
| Gene | Sequence | Annealing Temperature (°C) | Product Size (bp) |
|---|---|---|---|
| Hoxa10 | F-5’ACTAAGAGCAGCACGGTACG3’ R- 5’CCTTTGGAACTGCCTTGACTC3’ |
58 °C | 200 |
| 18s RNA | F- 5' AACCCGGTGAGCTCCCTCCC 3' R-5’TTCGAATGGGTCGTCGCCGC 3’ |
68 °C | 117 |
Immunohistochemistry
Immunohistochemistry was performed as described previously.[45,46] Briefly, paraffin sections were hydrated, antigens were retrieved using sodium citrate buffer (pH 6), blocking was done in 5% donkey serum (Jackson Immunology, Pennsylvania, USA), and the sections were probed overnight with primary goat anti-HOXA10 (Biomatik, Cambridge, Canada) diluted (1:250) in phosphate buffer saline (PBS). Negative controls were incubated with PBS instead of primary antibody. The next day, slides were washed with PBS and incubated with an antigoat biotinylated secondary antibody, followed by incubation in streptavidin- horseradish peroxidase (ABC Santa Cruz Biotechnology, Texas, USA). Detection was done using hydrogen peroxidase substrate and diaminobenzidine (Sigma Aldrich) as the chromogen. All sections were briefly counterstained with hematoxylin and mounted. Slides were viewed under a bright field microscope (Olympus, Tokyo, Japan) and representative areas were photographed.
Immunostaining Quantification
Immunostaining intensity was quantified using the “Fiji” version of ImageJ.[47] To quantify immunostaining for HOXA10 in the endometrium, we selected three different images showing the different areas of the same lesion and calculated the intensity. For individual cell types, namely stromal and epithelial cells, we randomly selected different areas of 2000 mm2 in stroma and epithelia, and the intensity was calculated. To quantify the nuclear and cytoplasmic staining of HOXA10, we performed a single-cell analysis. This intensity was further converted into optical density (OD) with the following formula in MS Excel:
OD = log (max intensity/mean intensity), where max intensity = 255 for 8-bit images.
Statistical Analysis
All experimental quantitative data were calculated in terms of mean and standard error mean (SEM), and statistical analysis was done by Kruskal–Wallis nonparametric analysis of variance testing, followed by Dunn's multiple comparison test. P value ≤0.05 was accepted as statistically significant.
RESULTS
Lesion Characteristics
As shown in Figure 1a, endometriosis was surgically induced by implanting a uterine fragment with intestinal mesentery in the mouse from which it came. Ectopic lesions were excised on days 10, 15, 20, 30, 45, 60, and 65 post-surgery. Following earlier studies, a single lesion grew progressively in size, and adhesions developed after day 30. In general, the lesions were cystic and white during the early stages, in some animals red lesions were seen. However, chocolate cysts or black lesions were not observed. Based on the extent of infiltration, these lesions were classified as peritoneal and superficial. In none of the animals, the lesions invaded the surrounding tissue even after remaining in the peritoneum for more than 2 months.
Lesion Histology
Irrespective of the time, glands and stroma were visible on the ectopic tissues. As time progressed, one of the glands acquired a cystic nature filled with clear fluid. No cells were ever visible in the cyst. In humans, endometriosis is classified based on epithelial cell pattern into well-differentiated, stromal, mixed, and undifferentiated types of endometriosis.[48] Herein, hematoxylin and eosin staining demonstrated that in almost 81% of animals (18/21 animals) the epithelium appeared columnar and resembled the well-differentiated type of endometriosis. In 19% (3/21 animals) of animals had mixed type where well differentiated and poorly differentiated epithelium was seen. Undifferentiated and stromal-type endometriotic lesions were not observed in any of the animals [Figure 1b].
Expression of HOXA10 Microribonucleic Acid in Endometriotic Tissues
Quantitative PCR was carried out with ectopic lesions acquired on days 15, 30, and 60 postsurgery, and the results were compared with eutopic endometrium of controls to determine the expression level of HOXA10 at the mRNA level. When the HOXA10 mRNA mean level was measured at day 15, it was found to be about 50% lower than controls. Conversely, when lesions were collected on days 30 and 60, HOXA10 expression levels were nearly 80% lower than controls [Figure 2a].
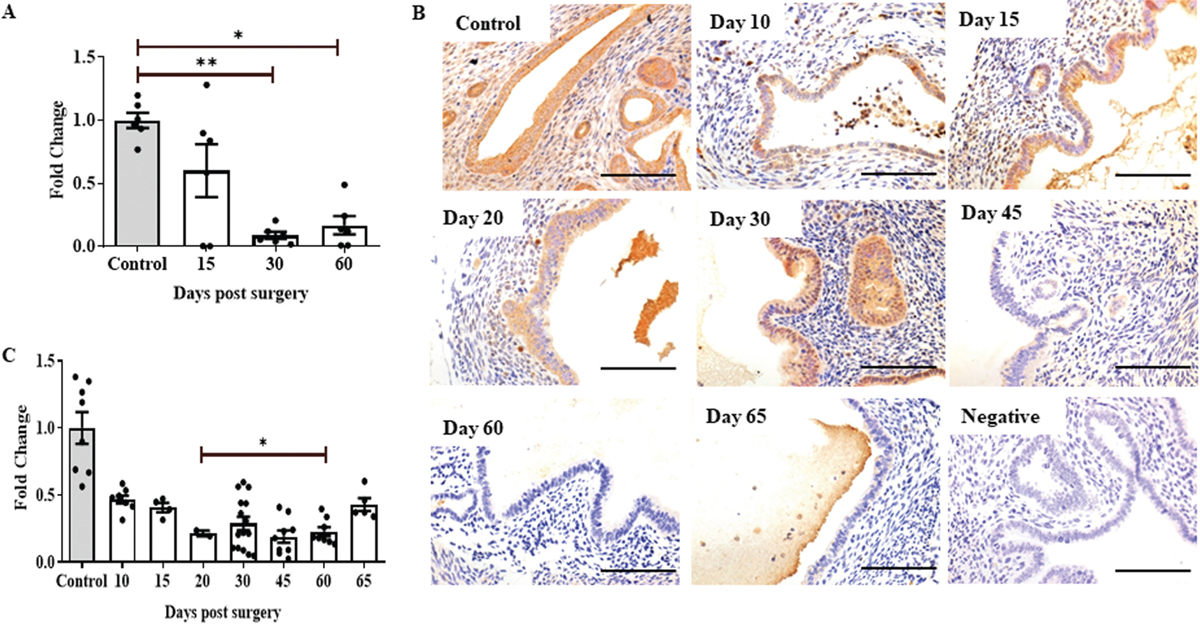
- Homeobox gene A10 (HOXA10) is downregulated in induced endometriosis in a temporally regulated manner. (a) Quantitative polymerase chain reaction (qPCR) result showing expression of HOXA10 at different time points in ectopic lesions and in eutopic endometrium of control mice. Each data point is one replicate (n = 3/time point). (b) Immunohistochemistry showing expression of HOXA10 in ectopic endometrium of mice at different time points (brown staining). Negative is control incubated without a primary antibody. Sections are counterstained with hematoxylin (blue staining); scale bar, 50 µm. (c) Immunostaining quantification for HOXA10 in the endometrium of control and ectopic lesions at different time points. Y axis showing fold change where control is taken as one. Mean ± standard error mean (SEM) for n = 3 control, n = 3 ectopic lesion per time point (days 10, 20, 30, 45, 60, and 65 post-surgery). Each point is a single replicate. *P < 0.05, **P < 0.01 were taken as statistically significant and are shown by horizontal bars.
Expression of HOXA10 Endometriosis during Lesion Development
To understand the changes in expression of HOXA10 during evolution of endometriosis, we performed immunohistochemistry on lesions excised on days 10, 15, 20, 30, 45, 60, and 65 postsurgery [Figure 2b]. Result shows that in the endometrium of mice without endometriosis, HOXA10 expression was largely nuclear in stromal cells, while both nuclear and cytoplasmic signals were detected in the epithelial cells. In endometriotic lesions, expression of HOXA10 appeared reduced in both epithelial and stromal cells at all the time points as compared to the endometrium of control mice. The negative control did not show any staining demonstrating the specificity of the reaction.
Quantitatively, as compared to the control endometrium, there was a significant reduction in HOXA10 in the endometriotic tissues across all the time points [Figure 2c]. On day 10, the expression reduced by almost 50% and remained low by day 15. By day 20 of lesion induction, the expression reduced further and was nearly 25% of the control values, which remained consistent until day 60. On day 65, the expression increased marginally as compared to day 60 [Figure 2c].
HOXA10 levels in Histological Subtypes of Endometriosis
According to the epithelial cell pattern, endometriosis is categorized into four types in humans: well-differentiated, stromal, mixed, and undifferentiated.[48] A well-differentiated, mixed type of endometriosis developed in the majority of the animals in this study. The expression of HOXA10 in these two distinct forms of endometriosis is also examined here [Figure 3]. As we can see in [Figure 3a], the expression of HOXA10 in well-differentiated and mixed types of endometriosis is nearly identical, while it is lower than in the control endometrium. In comparison to controls, the mean level of HOXA10 is quantitatively decreased by more than 50% in cases of well-differentiated and mixed endometriosis [Figure 3b]. Nonetheless, HOXA10 protein levels in well-differentiated and mixed types of endometriosis are nearly identical [Figure 3c].
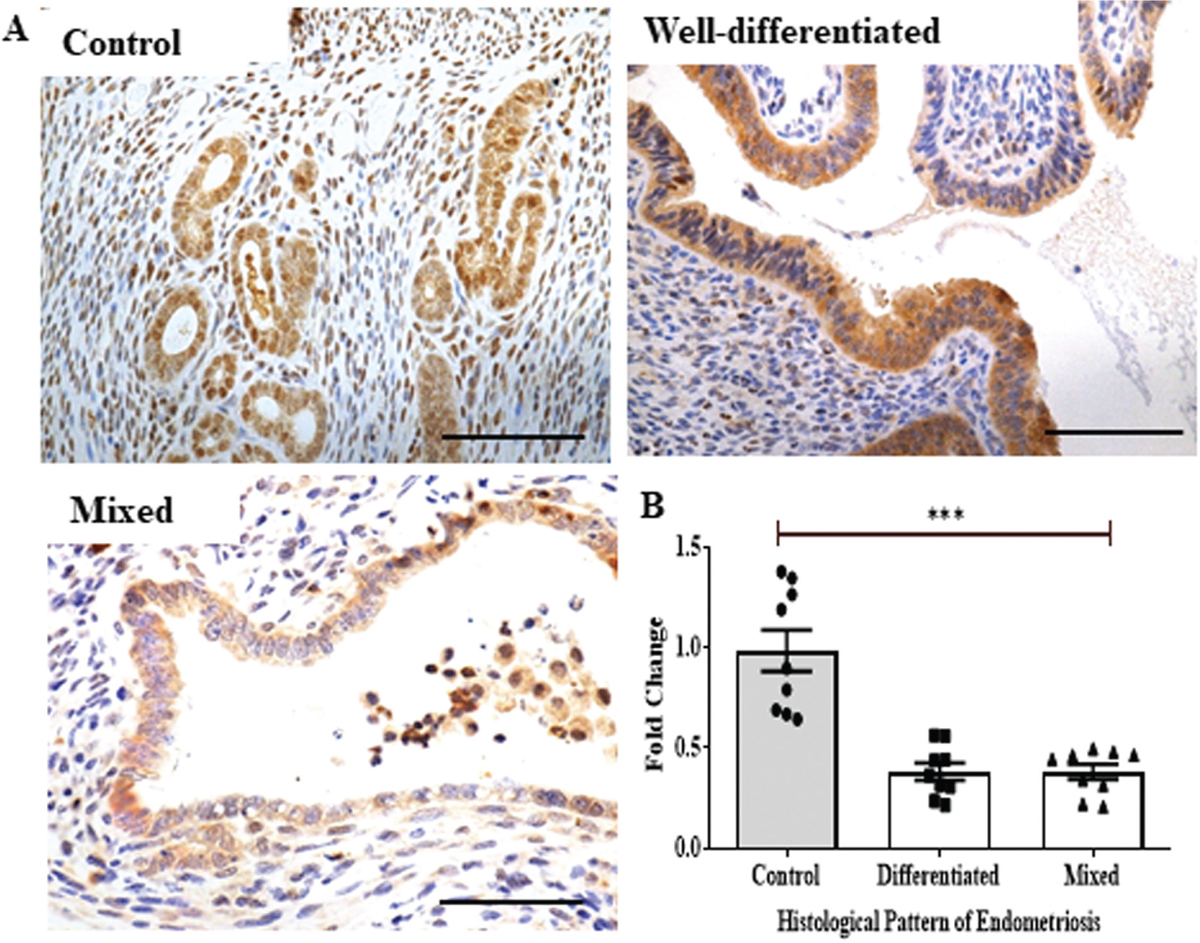
- Immunohistochemistry of Homeobox gene A10 (HOXA10) in histological sub-types of endometriosis. (a) Immunohistochemistry showing expression of HOXA10 in ectopic endometrium of mice (brown staining). Uterine sections are counterstained with hematoxylin (blue staining); scale bar, 50 µm. (b) Immunostaining quantification for HOXA10 in the endometrium of control and different types of endometriosis (well-differentiated and mixed). Y-axis showing fold change where control is taken as one. Mean ±standard error mean (SEM) for n = 3 control, n = 3 well-differentiated ectopic lesions, and n = 3 mixed lesions. Each data point is one replicate ***P < 0.001 was taken as statistically significant and is shown by horizontal bars.
Cell Type-specific Changes in Expression of HOXA10 during Endometriosis
To determine if there was any cell type difference in the expression of HOXA10 during lesion evolution, we analyzed the expression of HOXA10 in epithelial and stromal cells separately. In both epithelial and stromal cells, there was a reduction in the expression of HOXA10 at day 10 as compared to controls, but this was not statistically significant. From day 15 onward, there was more than a 50% reduction in the expression of HOXA10 in both epithelial and stromal cells when compared to controls [Figure 4a and 4b].
To address whether there was an equal reduction in both cell types or there was a preferential reduction in one cell type over other, we calculated the ratio of epithelium/stroma intensities of HOXA10 immunostaining. In the initial phase (day 10), although there was a reduction in overall expression of HOXA10, the ratio was 1, implying that the reduction was to a similar extent in both the cell types. However, as time progressed, the ratio reduced and was almost 50% on day 20 indicating that there was a more rapid decline in HOXA10 in the epithelium than in the stroma. However, after day 30 and until day 65 the epithelia-to-stromal ratio doubled, implying that after day 30, the stromal cells show a more rapid decline than epithelial cells [Figure 4c].
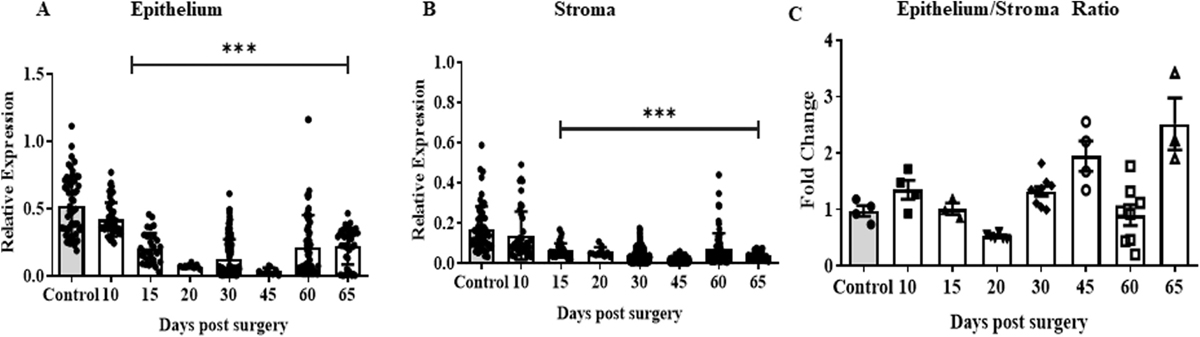
- Immunoquantification of HOXA10 in both epithelium and stroma irrespective of time. Quantification for HOXA10 immunostaining in epithelial (a) and stromal (b) cells of endometriotic lesions and control endometrium at different time points. (c) Ratio of HOXA10 in epithelial/stromal cells in control and ectopic lesions at different time points. Y axis showing fold change where the mean of control is taken as one and the relative levels in each replicate are calculated. Mean ± standard error mean (SEM) for n = 3 control, n = 3–9 ectopic lesion per time point (day 10, 20, 30, 45, 60, and 65 postsurgery). In a and b, each point is values in different areas of sections. In c each point is data of a single animal. ***P < 0.001 was taken as statistically significant and is shown by horizontal bars.
Nuclear–Cytoplasmic Shuttling of HOXA10 in Endometriosis
Since HOXA10 is a transcription factor, its ability to translocate to the nucleus is critical for this activity. Heat maps and their cognate immunohistochemistry show that the expression of HOXA10 was predominantly nuclear in stromal cells and both nuclear and cytoplasmic in epithelial cells [Figure 5a and b]. While the expression was reduced in both epithelial and stromal cells in endometriosis, the cytoplasmic HOXA10 was reduced to a greater extent than nuclear reduction. To address this temporally, we calculated the nuclear/cytoplasmic ratios in epithelial and stromal cells separately. In epithelial cells, on day 15, the nuclear-to-cytoplasmic ratio appeared similar to that of controls, indicating that there is no defect in HOXA10 shuttling. However, on day 30, the ratio increased significantly, implying that the HOXA10 is retained in the nucleus and later in the cytoplasm. As time progressed, on day 60, the ratio reduced and was even lower than controls, indicating that the nuclear translocation of HOXA10 is reduced, and there is more cytoplasmic retention [Figure 5c]. In stromal cells, nuclear/cytoplasmic ratio of HOXA10 expression was similar to controls on day 15; however, it significantly increased on days 30 and 60 indicating that in the stroma, the HOXA10 remained largely in the nucleus [Figure 5d].
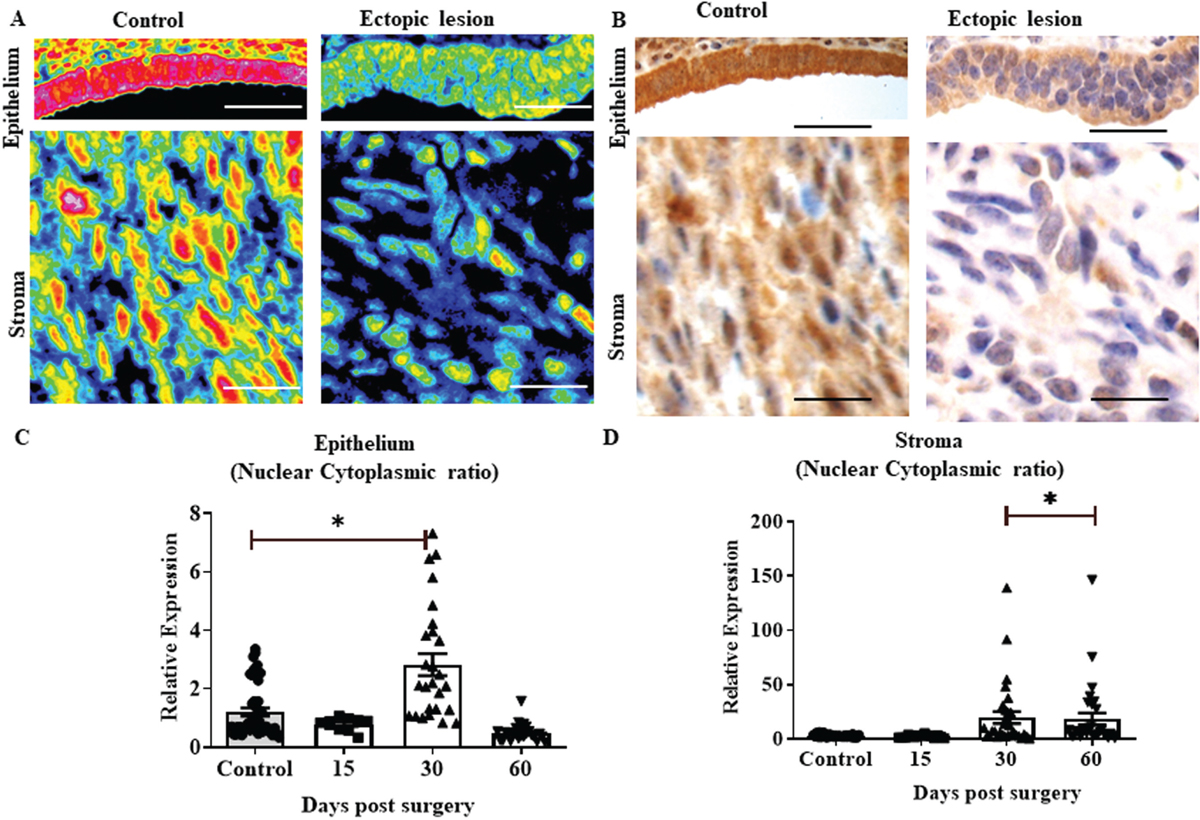
- Nucleo-cytoplasmic shuttling of HOXA10 in endometriosis. (a) Heat profiles of epithelium and stroma of control and ectopic lesions. The corresponding bright field images are shown in (b); scale bar, 50 µm. HOXA10 intensities were quantified separately in the nucleus and cytoplasm and the ratio was calculated. (c and d) Intensity ratios of nuclear/cytoplasmic HOXA10 in stromal and epithelial cells of control and ectopic lesions on different days. Y-axis showing relative expression of HOXA10 in control and ectopic lesions at days 15, 30, and 60. Mean ± standard error mean (SEM) for n = 10 control, n = 5 ectopic lesion per time point. In c and s each point is data from different areas of sections *P < 0.05 was taken as statistically significant and is shown by horizontal bars.
DISCUSSION
In this study, we surgically induced endometriosis in a mouse model and collected endometriosis lesions at different time points. We demonstrated that the expression of HOXA10 is reduced in endometriosis lesions at different time points. Additionally, the expression of HOXA10 was similarly reduced in well-differentiated or mixed types of endometriosis. Furthermore, we observed that the reduction in HOXA10 occurs more in stromal cells than in epithelial cells, and the nucleocytoplasmic shuttling was also affected in endometriosis.
According to available studies, expression of HOXA10 is altered in both eutopic and ectopic endometrium of women with endometriosis.[17,18,19,20] However, these studies are controversial, as some studies reported low expression of HOXA10 in endometriosis, while other studies have reported high expression of HOXA10 in endometriosis.[34] The reasons behind the controversy are still unclear. It is assumed that variability in results may be due to variations in method, sampling, timing, and phase of cycle.[49,50] Additionally, we do not know whether this reduction in expression is an inherent feature or a consequence of endometriosis. To address these issues, we induced endometriosis in young female mice of the same age and collected the tissues in the phase of the cycle. The results demonstrate that the expression of HOXA10 is low in endometriosis lesions of the mouse model irrespective of time points. These observations imply that HOXA10 is reduced in endometriosis, perhaps as a consequence of endometriosis. Further, this reduction is progressive, implying that the early lesions might be responsive to HOXA10 signaling, and the effects may be exacerbated as the lesion progresses.
In humans, endometriosis is classified by different methods, with the most common being based on the location (e.g., peritoneal endometriosis, ovarian endometriosis, etc.) or the extent of infiltration of lesions, such as superficial lesions and deep infiltrating lesions.[1,5] HOXA10 is known to be reduced in peritoneal and ovarian endometriosis.[25,32,51,52,53,54] However, a comparative study of the extent of reduction in different types of lesions or stages of lesion development is lacking. In the animal model, we report that the leaves of HOXA10 progressively reduce in the peritoneal superficial type of endometriosis. In humans, endometriosis is histologically categorized into well-differentiated, stromal, mixed, and undifferentiated types.[48] The difference in levels of HOXA10 in these histologically distinct subtypes is not known. In our model, the majority of lesions were of the well-differentiated type; mixed phenotypes were only seen in a small number of animals. None of the animals exhibited stromal or undifferentiated forms of endometriosis. We did not find a significant difference in the expression of HOXA10 in these two types of endometriosis suggesting that the histological grades of endometriosis evolve independently of HOXA10, and other factors might be involved in this pathogenesis.
In the endometrium, HOXA10 is known to have its major effects on stromal cells than epithelial cells.[14,55] In the mouse model of endometriosis, we observed that in the initial stages of lesion development, HOXA10 is rapidly lost in epithelial cells but eventually stabilized with lesion growth, while the stromal cell expression is continuously reduced with the evolution of the lesion. This suggests that in endometriosis, HOXA10 loss is more exacerbated in the stroma than in the epithelium. Indeed, there are many stromal cell defects reported in endometriosis, including defective decidualization.[19,56,57] Of these, we and others have shown that decidualization is a key process regulated by HOXA10 in stromal cells.[13,15,58]
Nucleocytoplasmic translocation of proteins is a complex process that requires the contribution and interaction of many proteins.[59] Transcription factors, such as HOXA10, are well known to shuttle between the nucleus and cytoplasm, as they contain DNA-binding domains that bind with the promoter region of DNA to either activate or inhibit the transcription of target genes.[60] In the endometrium, HOXA10 expression is predominantly nuclear in both epithelial and stromal cells and their ratio is maintained. However, in endometriosis lesions, the ratio is disrupted with more nuclear expression than cytoplasmic, indicating that nucleocytoplasmic shuttling of the HOXA10 protein is affected in endometriosis. The extended nuclear localization of HOXA10 would imply that the HOXA10-driven transcriptome will be disrupted in the two cell types of endometriosis. Further, experimental studies must be conducted to elucidate the relationship between HOXA10 compartmentalization, nucleocytoplasmic shuttling, HOXA10-regulated transcriptome, and the etiopathogenesis of endometriosis.
The consequence of loss of HOXA10 in endometriosis awaits experimental investigations. In the normal endometrium, a large proportion of progesterone effects are mediated via HOXA10[14,61,62]; therefore, the progesterone resistance observed in endometriosis may be due to the loss of HOXA10. Also, the loss of HOX10 is associated with the overexpression of inflammatory factors.[13,15] Since these factors are also increased in women with endometriosis, we propose that the inflammatory phenotype in women with endometriosis is at least in part contributed by the loss of HOXA10. Finally, the loss of HOXA10 leads to endometrial epithelial and stromal cell proliferation.[26] The progressive growth of endometriotic lesions, in part could likely be contributed by the loss of HOXA10. It will be of interest to study these phenotypes in mice that lack HOXA10 to address how this gene contributes to the pathogenesis of endometriosis.
CONCLUSION
HOXA10 expression is progressively downregulated in both epithelial and stromal cells of endometriosis tissues, and there is defective nucleocytoplasmic shuttling of the HOXA10 protein. Such dysregulation of a key transcription factor can contribute to the pathogenesis of endometriosis. It will be imperative to develop drugs that simulate or are agonists of HOXA10, which might help in the treatment and/or management of endometriosis.
REFERENCES
- Endometriosis: epidemiology, classification, pathogenesis, treatment and genetics (review of literature) Int J Mol Sci. 2021;22:10554.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting the formation of estrogens for treatment of hormone dependent diseases-current status. Front Pharmacol. 2023;14:1-16.
- [CrossRef] [PubMed] [Google Scholar]
- Retrograde menstruation in healthy women and in patients with endometriosis. J Am Coll Obstet Gynecol. 1984;64:151-4.
- [Google Scholar]
- Extracellular vesicles in embryo implantation and disorders of the endometrium. Am J Reprod Immunol. 2021;85:e13360.
- [Google Scholar]
- Understanding the pathogenesis of endometriosis. in Recent update in Endometriosis. In: Rozati R, Nayar KD, Jain K. (eds), Indian Fertility Society, Evangel Publishing Pvt Ltd. 2022;1
- [Google Scholar]
- Endometrial and placental stem cells in successful and pathological pregnancies. J Assist Reprod Genet. 2023;40:1509-22.
- [CrossRef] [PubMed] [Google Scholar]
- Pathogenesis of endometriosis: the genetic/epigenetic theory. Fertil Steril. 2019;111:327-40.
- [CrossRef] [PubMed] [Google Scholar]
- Auto/cross-regulation of Hoxb3 expression in posterior hindbrain and spinal cord. Dev Biol. 2002;252:287-300.
- [CrossRef] [PubMed] [Google Scholar]
- The emerging role of menstrual-blood-derived stem cells in endometriosis. Biomedicines. 2023;11:1-10.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of HOXA10 gene in women with endometriosis: a systematic review. Int J Mol Sci. 2023;24:12869.
- [CrossRef] [PubMed] [Google Scholar]
- Homeobox genes in endometrium: from development to decidualization. Int J Dev Biol. 2020;64:227-37.
- [CrossRef] [PubMed] [Google Scholar]
- The role of the Hoxa10/HOXA10 gene in the etiology of endometriosis and its related infertility: a review. J Assist Reprod Genet. 2010;27:701-10.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of decidualization, interleukin-11 and interleukin-15 by homeobox A 10 in endometrial stromal cells. J Reprod Immunol. 2010;85:130-9.
- [CrossRef] [PubMed] [Google Scholar]
- HOXA10 signals on the highway through pregnancy. J Reprod Immunol. 2009;83:72-8.
- [CrossRef] [PubMed] [Google Scholar]
- Decrease in expression of HOXA10 in the decidua after embryo implantation promotes trophoblast invasion. Endocrinology. 2017;158:2618-33.
- [CrossRef] [PubMed] [Google Scholar]
- The role of Hox genes in female reproductive tract development, adult function, and fertility. Cold Spring Harb Perspect Med. 2016;6:a023002.
- [CrossRef] [PubMed] [Google Scholar]
- HOXA-10 gene expression in ectopic and eutopic endometrium tissues: does it differ between fertile and infertile women with endometriosis? Eur J Obstet Gynecol Reprod Biol. 2019;233:43-8.
- [Google Scholar]
- HOXA10 expression in ectopic endometrial tissue. Fertil Steril. 2006;85:1386-90.
- [CrossRef] [PubMed] [Google Scholar]
- Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod. 2007;13:323-32.
- [CrossRef] [PubMed] [Google Scholar]
- DNA methylation of HOXA10 in eutopic and ectopic endometrium. Hum Reprod. 2014;29:1906-11.
- [CrossRef] [PubMed] [Google Scholar]
- Expression and significance of ROCK1 and HOXA10 in ectopic and eutopic endometrium of patients with endometriosis. J New Med. 2019;50:763-7.
- [Google Scholar]
- Aberrant endometrial DNA methylome of homeobox A10 and catechol-O-methyltransferase in endometriosis. J Assist Reprod Genet. 2017;34:409-15.
- [CrossRef] [PubMed] [Google Scholar]
- Epigenetic dynamics of HOXA10 gene in infertile women with endometriosis. Reprod Sci. 2019;26:88-96.
- [CrossRef] [PubMed] [Google Scholar]
- Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Res. 2018;28:819-32. y.
- [CrossRef] [PubMed] [Google Scholar]
- Aberrant expression of lncRNA (HOXA11-AS1) and homeobox A (HOXA9, HOXA10, HOXA11, and HOXA13) genes in infertile women with endometriosis. Reprod Sci. 2018;25:654-61.
- [CrossRef] [PubMed] [Google Scholar]
- Loss of HOXA10 causes endometrial hyperplasia progressing to endometrial cancer. J Mol Endocrinol. 2022;69:431-44.
- [CrossRef] [PubMed] [Google Scholar]
- HOXA10 controls proliferation, migration and invasion in oral squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8:3613-23.
- [Google Scholar]
- Identification of HOX signatures contributing to oral cancer phenotype. Sci Rep. 2022;12:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- The role of mir-27b-3p/hoxa10 axis in the pathogenesis of endometriosis. Ann Palliat Med. 2021;10:3162-70.
- [CrossRef] [PubMed] [Google Scholar]
- Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol. 2005;193:371-80.
- [CrossRef] [PubMed] [Google Scholar]
- Methylation analysis of HOXA10 regulatory elements in patients with endometriosis. BMC Res Notes. 2018;11:1-5.
- [CrossRef] [PubMed] [Google Scholar]
- Methylation of the HOXA10 homeobox gene promoter is associated with endometrial cancer: a pilot study. J Obstet Gynaecol (Lahore). 2013;33:519-20.
- [CrossRef] [PubMed] [Google Scholar]
- HOXA10 DNA methylation level in the endometrium women with endometriosis: a systematic review. Biology (Basel). 2023;12:474.
- [CrossRef] [PubMed] [Google Scholar]
- The relationship between the expression levels of miR-135a and HOXA10 gene in the eutopic and ectopic endometrium. Int J Reprod Biomed. 2018;16:501-6.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic alterations of HOXA 10 and their effect on the severity of endometriosis in a Taiwanese population. Reprod Biomed Online. 2008;16:416-24.
- [CrossRef] [PubMed] [Google Scholar]
- Research advances in endometriosis-related signaling pathways: a review. Biomed Pharmacother. 2023;164:114909.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of inflammation pathways and inflammasome by sex steroid hormones in endometriosis. Front Endocrinol (Lausanne). 2020;10:935.
- [Google Scholar]
- Animal models for research on endometriosis Department of Obstetrics and Gynecology, Tottori University Faculty of Medicine, Yonago, 683-3 Genomic function of estrogen receptor β (BETA) in endometriosis 4. Murine endometriosis models 4. 1. Homo. Front Biosci. 2021;13:37-53.
- [CrossRef] [PubMed] [Google Scholar]
- Translational animal models for endometriosis research: a long and windy road. Ann Transl Med. 2018;6:431.
- [CrossRef] [PubMed] [Google Scholar]
- Spatial and temporal changes in the expression of steroid hormone receptors in mouse model of endometriosis. J Assist Reprod Genet. 2020;37:1069-81.
- [CrossRef] [PubMed] [Google Scholar]
- Estrogen is essential but not sufficient to induce endometriosis. J Biosci. 2017;42:251-63.
- [CrossRef] [PubMed] [Google Scholar]
- Mouse model for endometriosis is characterized by proliferation and inflammation but not epithelial-to-mesenchymal transition and fibrosis. J Biosci. 2020;45:105.
- [CrossRef] [PubMed] [Google Scholar]
- Mouse model of surgically-induced endometriosis by auto-transplantation of uterine tissue. J Vis Exp 2012:e3396.
- [CrossRef] [PubMed] [Google Scholar]
- Oviductal glycoprotein 1 (OVGP1) is expressed by endometrial epithelium that regulates receptivity and trophoblast adhesion. J Assist Reprod Genet. 2018;35:1419-29.
- [CrossRef] [PubMed] [Google Scholar]
- LHX2 in germ cells control tubular organization in the developing mouse testis. Exp Cell Res. 2023;425:113511.
- [CrossRef] [PubMed] [Google Scholar]
- Extra-oviductal expression of oviductal glycoprotein 1 in mouse: detection in testis, epididymis and ovary. J Biosci. 2017;42:69-80.
- [CrossRef] [PubMed] [Google Scholar]
- Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9:676-82.
- [CrossRef] [PubMed] [Google Scholar]
- Histological classification of endometriosis as a predictor of response to treatment. Int J Gynaecol Obstet. 2003;82:31-40.
- [CrossRef] [PubMed] [Google Scholar]
- Human Endometriosis Tissue Microarray Reveals Site-specific Expression of Estrogen Receptors, Progesterone Receptor, and Ki67. Appl Immunohistochem Mol Morphol. 2019;27:491-500.
- [CrossRef] [PubMed] [Google Scholar]
- The elusive and controversial roles of estrogen and progesterone receptors in human endometriosis. Am J Transl Res. 2014;6:104-13.
- [Google Scholar]
- Endometriosis located proximal to or remote from the uterus differentially affects uterine gene expression. Reprod Sci. 2016;23:186-91.
- [CrossRef] [PubMed] [Google Scholar]
- HOXA-10 expression in the mid-secretory endometrium of infertile patients with either endometriosis, uterine fibromas or unexplained infertility. Hum Reprod. 2009;24:3180-7.
- [CrossRef] [PubMed] [Google Scholar]
- Differential expression of genes from the homeobox A cluster in deep endometriotic nodules and peritoneal lesions. Fertil Steril. 2010;94(6):1995-2000.
- [CrossRef] [PubMed] [Google Scholar]
- A preliminary communication: Ongoing study on HOXA10 methylation profile of endometriosis patients with infertility. J Endometr. 2016;8:106-10.
- [CrossRef] [PubMed] [Google Scholar]
- Embryo implantation: war in times of love. Endocrinology. 2018;159:1188-98.
- [CrossRef] [PubMed] [Google Scholar]
- FKBP4 is regulated by HOXA10 during decidualization and in endometriosis. Reproduction. 2012;143:531-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prokineticin 1, homeobox A10, and progesterone receptor messenger ribonucleic acid expression in primary cultures of endometrial stromal cells isolated from endometrium of healthy women and from eutopic endometrium of women with endometriosis. Fertil Steril. 2010;94:2558-63.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of HOXA10 target genes in human endometrial stromal cells by RNA-seq analysis. Acta Biochim Biophys Sin (Shanghai). 2021;53:365-71.
- [CrossRef] [PubMed] [Google Scholar]
- The rules and functions of nucleocytoplasmic shuttling proteins. Int J Mol Sci. 2018;19:1-17.
- [CrossRef] [PubMed] [Google Scholar]
- Nucleocytoplasmic shuttling of transcription factors. Cell Mol Life Sci. 2000;57:1193-206.
- [CrossRef] [PubMed] [Google Scholar]
- Gene expression profiling reveals progesterone-mediated cell cycle and immunoregulatory roles of Hoxa-10 in the preimplantation uterus. Mol Endocrinol. 2003;17:610-27.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of homeobox A10 expression in the primate endometrium by progesterone and embryonic stimuli. Reproduction. 2007;134:513-23.
- [CrossRef] [PubMed] [Google Scholar]







