Translate this page into:
Impact of intracytoplasmic morphologically selected sperm injection versus intracytoplasmic sperm injection on in vitro fertilization outcome in cases of poor sperm morphology or sperm deoxyribonucleic acid damage
Address for correspondence: Dr Divyaasha Walia, A 69 A, Sector 40, Noida, Uttar Pradesh, India. E-mail : drdivyaasha@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Devi MG, Sharma M, Goswami G,Walia D. Impact of intracytoplasmic morphologically selected sperm injection versus intracytoplasmic sperm injection on in vitro fertilization outcome in cases of poor sperm morphology or sperm deoxyribonucleic acid damage. Fertil Sci Res 2023;10:210-8.
Abstract
This study airms to asses intracytoplasmic morphologically selected sperm injection (IMSI) compared to intracytoplasmic sperm injection (ICSI) in cases of male factor infertility. This article compares IMSI and ICSI in patients with poor sperm morphology or a raised deoxyribonucleic acid (DNA) fragmentation index. After meeting the inclusion criteria, 134 couples were included in the study and randomized into two groups: in vitro fertilization (IVF)-ICSI and IVF-IMSI. The results showed a higher fertilization rate (77.2 vs 54.04%) and a higher quality embryo rate (61.2 vs 40.9%) in IMSI cycles compared to ICSI cycles, and these differences were statistically significant (P value 0.009). IMSI has shown better fertilization rates compared to ICSI in patients with poor morphology. The difference in biochemical pregnancy rates (56.72 vs 34.3%) between the two groups was statistically significant (P value 0.009). This study concluded that IMSI had a higher likelihood of resulting in pregnancy in couples with male factor infertility.
Keywords
In vitro fertilization
Intracytoplasmic morphologically selected sperm injection
Intracytoplasmic sperm injection
INTRODUCTION
Infertility is characterized by the inability to get pregnant after 12 months of unprotected sexual activity.[1] Infertility is thought to affect around 48 million couples in India[2] and 186 million couples worldwide.[3,4]
Infertility issues affect one in six couples globally at least once during their reproductive lives.[2,3,4]
Ethics: This study was approved by the Ethical Committee of Indian fertility society (IFS) Delhi.
Ethical number: F.1/IEC/IFS/2022No.30
Methodology
Affiliation: Ridge IVF Centre, Malka Ganj, Delhi.
Objective
To compare the fertilization rate and embryo quality between intracytoplasmic morphologically selected sperm injection (IMSI) and intracytoplasmic sperm injection (ICSI)
To compare the biochemical pregnancy rate of IMSI and ICSI among infertile couples with sperm deoxyribonucleic acid (DNA) damage and/or poor sperm morphology
To correlate sperm fragmentation index with sperm morphology
Inclusion Criteria
Couples with infertility undergoing assisted reproductive technique (ART) procedure
Couples undergoing IVF-ICSI/IMSI
Maternal age ≤38 years
DNA fragmentation index >15%
Sperm morphology <4% (Krugers Criteria)
Anti mullerian hormone (AMH) ≥1 ng/mL
Exclusion Criteria
History of associated endocrine disorders or other medical conditions (hypertension, diabetes, endometriosis, etc.)
Abnormal uterine factors diagnosed by vaginal sonography, hysteroscopy, hysterosalpingography, or previous uterine surgery
Cases requiring percutaneous epididymal sperm aspiration(PESA)/testicular sperm extraction(TESE)/testicular sperm aspiration(TESA)/microTESE
Study design: Prospective randomized observational comparative study.
Duration of study: Six months, January 2023 to June 2023.
Sample size: A total of 134 patients divided into two groups (of 67 each) of IVF-ICSI and IVF-IMSI.
The couples attending Ridge IVF gave written consent before being included in the study.
DNA = deoxyribonucleic acid, ICSI = intracytoplasmic sperm injection, IMSI = intracytoplasmic morphologically selected sperm injection.
Randomization
Patients accepted for the study were randomized using a simple computer-based randomization to ICSI or IMSI groups.%
Methods Used
Semen analysis and semen preparation: WHO 2010 criteria, morphology by strict Kruger's criteria.
Patients produced a semen sample after 3 to 5 days of sexual abstinence through masturbation on the day of oocyte retrieval oocyte pick up (OPU). All patients were required to produce the sample in the sperm wash medium to get good viable sperm. After liquefaction for 30 minutes, the sample was analyzed as per the 5th edition of WHO manual.[5] Volume, concertation, motility, agglutination, presence of round cells, and morphology were assessed before semen preparation. Sperm motility was assessed in 200 sperm and presented as a percentage of progressively motile sperm (amount of rapid progressive in addition to slow progression sperm). Viability was evaluated using Eosin-Nigrosin staining. For the evaluation of sperm morphology, strict Kruger criteria were employed. Sperm preparation was done using simple wash plus density gradient method.
For IMSI, motile sperm organelle morphology examination (MSOME) criteria were used. Bartoov et al.[14] developed a new method for real-time evaluation of sperm morphology, the MSOME. MSOME is accomplished by utilizing an inverted light microscope equipped with high-power Nomarski optics, enhanced by digital imaging to achieve a magnification above 6000×. Two forms of spermatozoa observed at MSOME were considered in this study: normal spermatozoa and spermatozoa with large nuclear vacuoles. A spermatozoon was classified as morphologically normal when it exhibited a normal nucleus as well as acrosome, post-acrosomal lamina, neck, and tail, besides not presenting cytoplasm around the head.
DNA Fragmentation Index (DFI): It was conducted using sperm chromatin dispersion (SCD) test method with the sperm chromatin kit uded was Cryolab International, (UK).
Principle: Sperm with fragmented DNA fail to produce the characteristic halo of dispersed DNA loops that is observed in sperm with nonfragmented DNA.
The method used in this study involved diluting semen samples with phosphate-buffered saline to obtain a sperm concentration that ranged between 5 and 10 million. The suspensions were mixed with 1% agarose to get a final agarose concentration of 0.7% at 37°C. Aliquots of 25 µL of the above mixture were pipetted onto a precoated agarose glass slide (0.65%) and covered with a glass coverslip (22 × 22 mm). The slides were then allowed to solidify for 5 minutes at 4°C. Coverslips were then gently removed, and the glass slides were immersed horizontally in a tray containing freshly prepared denaturation solution (0.08 N HCl) for 7 minutes at room temperature. Denaturation was then stopped by transferring the slides to another tray containing 10 mL of fresh lysis buffer (0.4 M Tris, 0.4 M dithiothreitol (DTT), 50 mM ethylene diamine tetra acetic acid (EDTA), 0.3% sodium dodecyl sulfate (SDS), and 1% Triton X‐100) and incubated for 25 minutes at room temperature. Slides were then thoroughly washed with distilled water, followed by dehydration for 2 minutes in each of 70, 90, and 100% ethanol and subsequently air dried. The dehydrated slides were stained with Giemsa and observed under bright-field microscopy for halos. The degree of DNA dispersion was assessed by observing the relative halo size under bright-field microscopy (Zeiss Axioskop 2 plus; Carl Zeiss, Gottingen, Germany). A minimum of 500 spermatozoa per sample was evaluated. In brief, four different dispersion patterns based on halo size were observed under bright-field microscopy: (i) sperm nuclei with big halo, (ii) sperm nuclei with medium-sized halo, (iii) sperm nuclei with very small halo, and (iv) sperm nuclei without halo. Sperms with large- and medium-sized halos are considered to be normal or nonfragmented, whereas sperms with small-sized halo or no halo are considered to have significant DNA fragmentation following acid denaturation and removal of nuclear proteins.[6]
Interpretation of dfi
<15% DFI = good fertility potential (excellent sperm)
≥15% DFI = clinically significant[7]
Intracytoplasmic Sperm Injection
After sperm preparation using both simple wash and density gradient methods, an optical magnification of 200× to 400× is used to examine the sample. The best morphologically “normal looking” motile spermatozoa were selected, and, based on their major morphology, they were injected into oocytes.
Intracytoplasmic Morphologically Selected Sperm Injection
Bartoov et al.[14] in 2003 developed a method for the real-time evaluation of human spermatozoa at high magnification called “MSOME”. It is performed using an inverted microscope equipped with Normarski interference contrast optics, which enables observation at high magnification (>6000×) compared to the 200 to 400× observed conventional ICSI.[5,6] This criteria is used for sperm selection in IMSI, which assesses a spermatozoon as morphologically normal when it exhibited a normal nucleus (smooth, symmetric, and oval nucleus, width 3.28 ± 0.20 µm, length 4.75 ± 0.20 µm/absence of vacuoles occupying >4% of nuclear area), as well as acrosome, post-acrosomal lamina, neck, and tail, besides not presenting cytoplasm around the head.
GRADING: EMBRYO
In order to transfer the embryos, they were cultured for 3 days (cleavage stage). The Istanbul consensus was used to grade the embryos.[8] High-quality embryos at the cleavage stage (grade A) were those that possessed all of the following characteristics: 4 cells on day 2 or 8 to 10 cells on day 3, 10% fragmentation, symmetric blastomeres, absence of multinucleation, colorless cytoplasm with moderate granulation and no inclusions, absence of perivitelline space granularity, and absence of zona pellucida dysmorphisms [Figure 1].
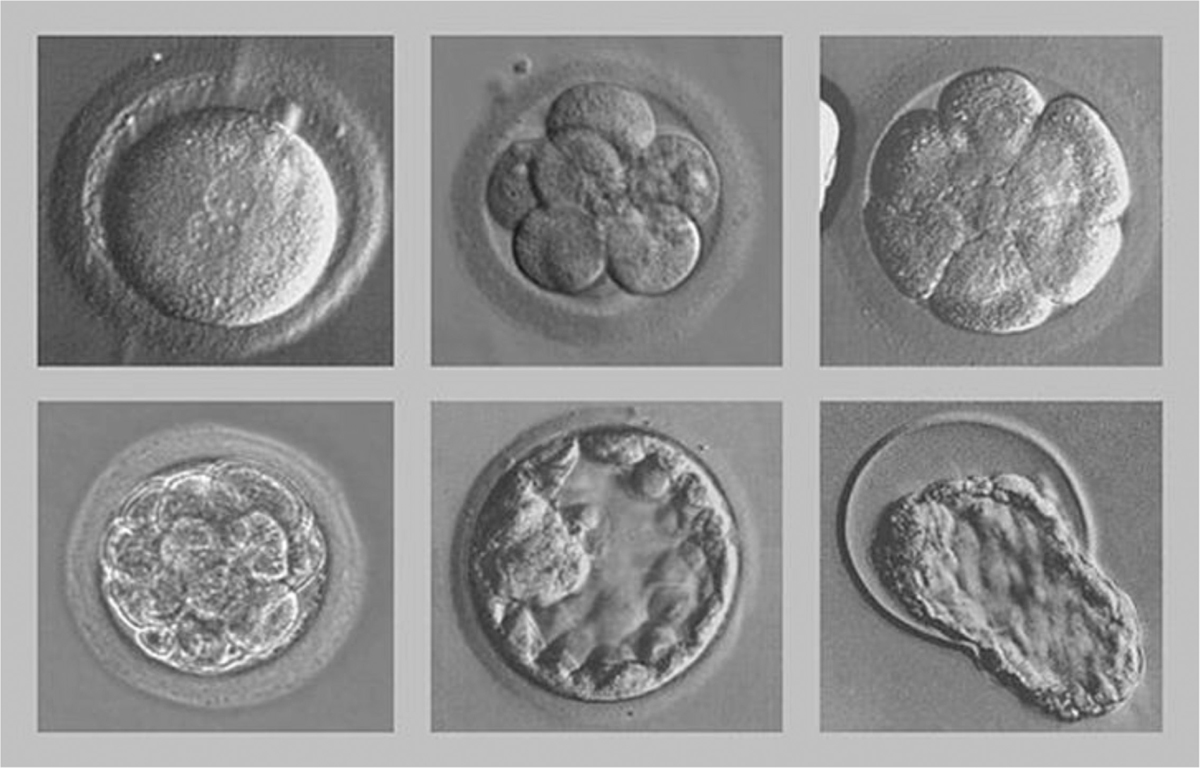
- Stage of development of embryos.
Results and Discussion
The study was conducted at Ridge IVF Centre (Malka Ganj, Delhi) between January till June 2023. A total of 134 couples meeting all the inclusion criteria were included in the study. They were divided into two groups: IVF–ICSI and IVF-IMSI. The outcomes in terms of fertilization rate, biochemical pregnancies, and the quality of embryo transferred between both the groups were analyzed, and the results are presented in the following tables.
In the study, half of the patients underwent ICSI, and the other half underwent IMSI each [Table 1, Figure 2].
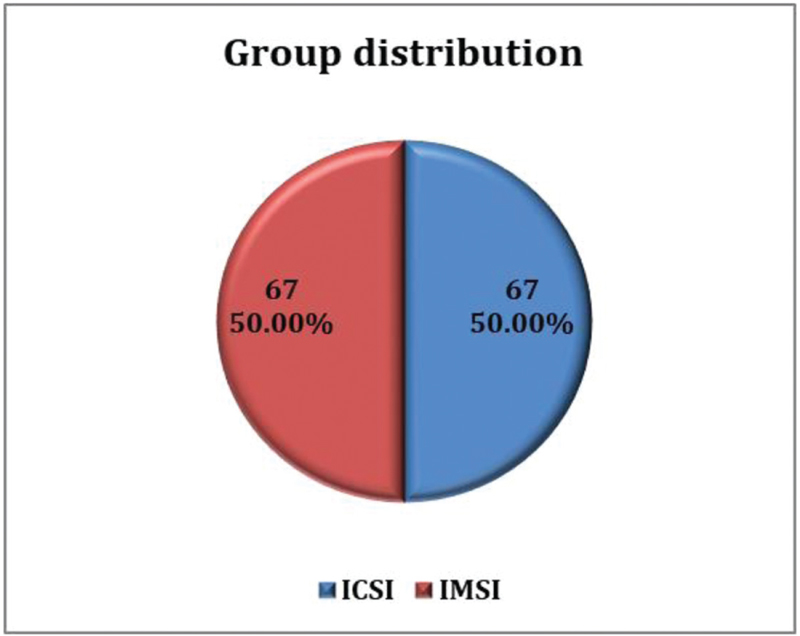
- Group distribution. ICSI = intracytoplasmic sperm injection, IMSI = intracytoplasmic morphologically selected sperm injection
| Group | Frequency | Percentage |
|---|---|---|
| ICSI | 67 | 50 |
| IMSI | 67 | 50 |
| Total | 134 | 100 |
ICSI = intracytoplasmic sperm injection, IMSI = intracytoplasmic morphologically selected sperm injection
Demographic Parameters
Age of husband of 74 (55.22%) cases was 31 to 35 years, 44 (32.84%) cases was 36 to 40 years, 15 (11.19%) cases was 26 to 30 years, and 1 (0.75%) case was 41 to 45 years. Mean value of age of husband (years) of study subjects was 34.3 ± 3.2 with a median (25th–75th percentile) of 34 (32–36) [Table 2, Figure 3].
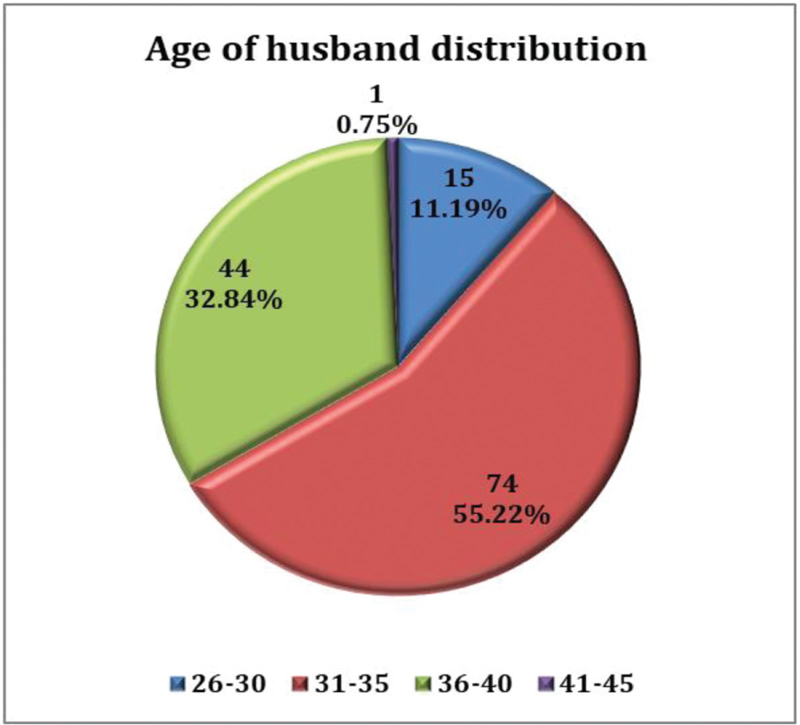
- Age of husband distribution.
| Age of Husband (Years) | Number | Percentage |
|---|---|---|
| 26-30 | 15 | 11.19 |
| 31-35 | 74 | 55.22 |
| 36-40 | 44 | 32.84 |
| 41 -45 | 1 | 0.75 |
| Mean ± SD | 34.3 ± 3.2 | |
| Median (25th-75th percentile) | 34 (32-36) | |
| Range | 26-44 | |
No correlation was observed between sperm morphology and the DNA fragmentation index [Table 3, Figure 4].
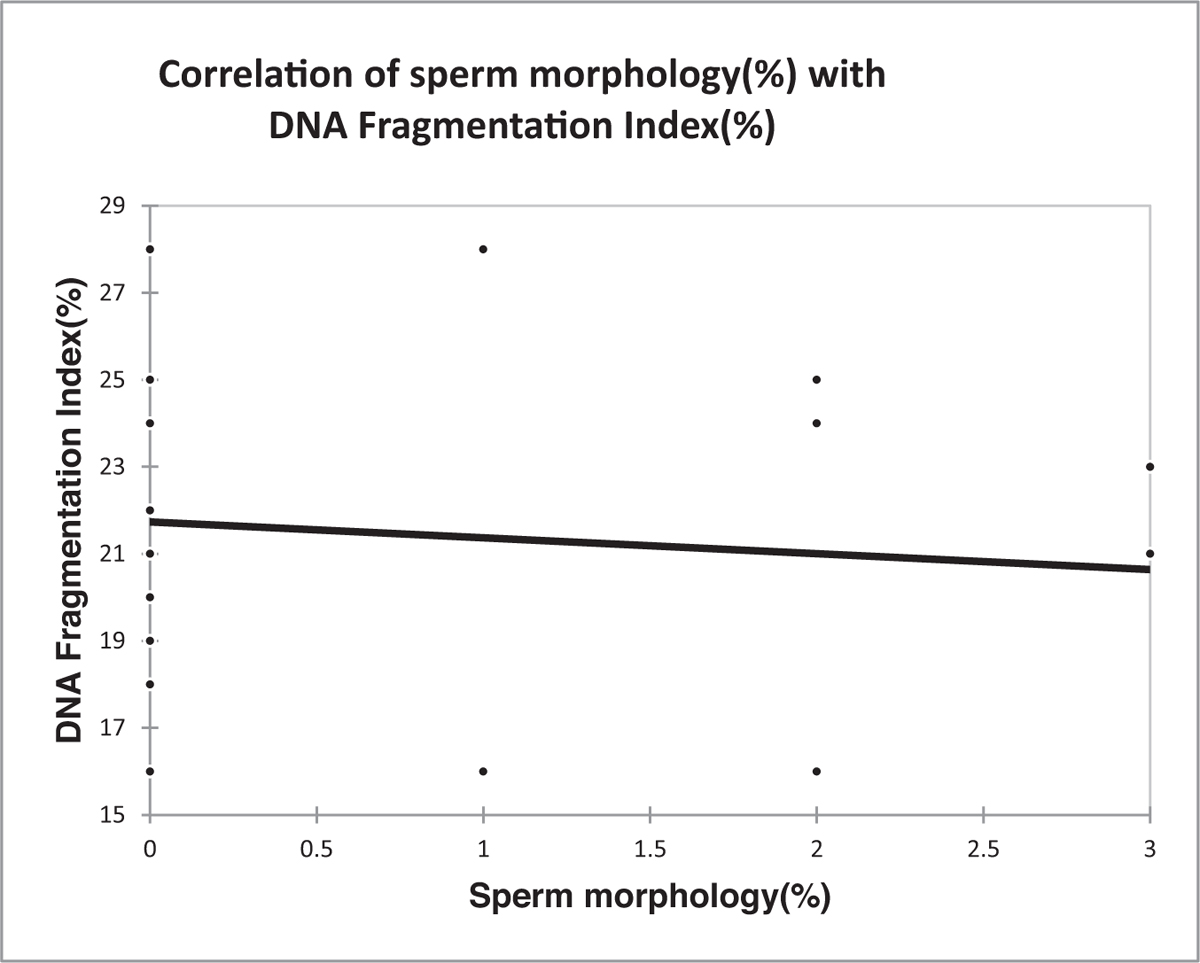
- Correlation of sperm morphology (%) with DNA fragmentation index (%). DNA = deoxyribonucleic acid
| Variables | DNA Fragmentation Index (%) |
|---|---|
| Sperm Morphology (%) | |
| Correlation coefficient | -0.087 |
| P value | 0.723 |
Spearman rank correlation coefficient. DNA = deoxyribonucleic acid
The median (25th–75th percentile) number of grade A embryos in IMSI was 5 (3–8), which was significantly higher compared to ICSI [2 (1–4)] (P value <0.0001) [Table 4, Figure 5].
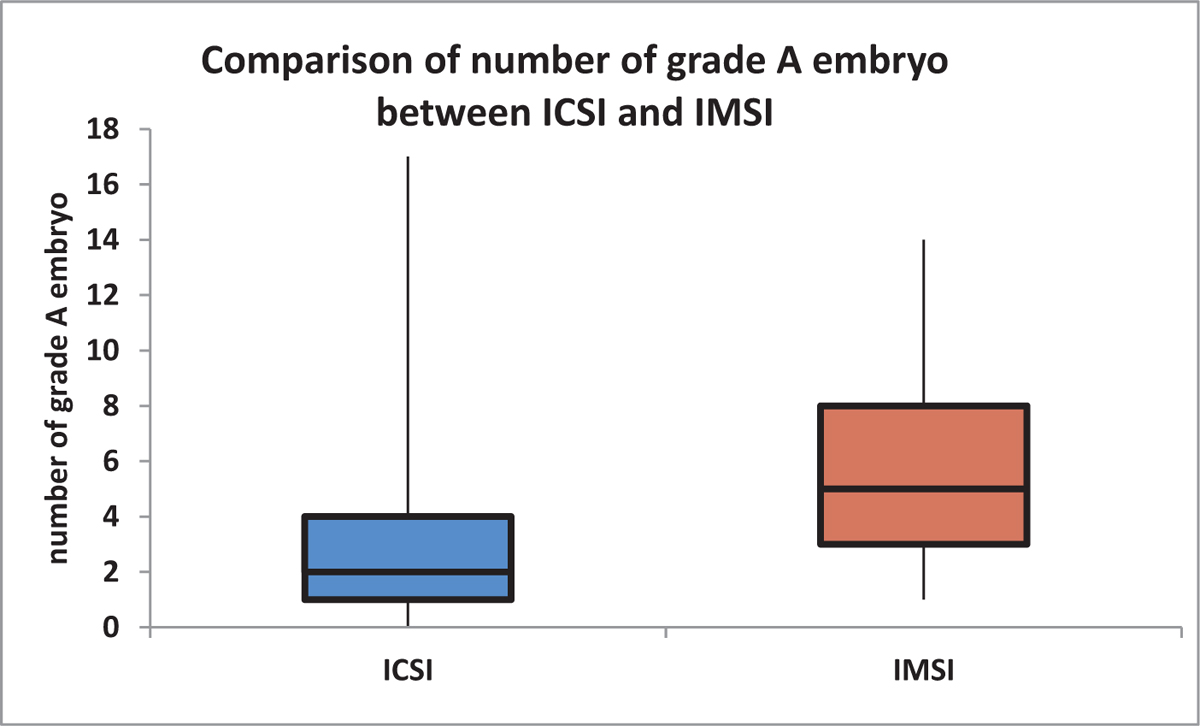
- Comparison of number of grade A embryo between ICSI and IMSI. (nonparametric variable, box–whisker plot). ICSI = intracytoplasmic sperm injection, IMSI = intracytoplasmic morphologically selected sperm injection
| Number of Grade A Embryo | ICSI (n = 67) | IMSI (n = 67) | Total | P Value |
|---|---|---|---|---|
| Mean ± SD | 2.93 ±2.78 | 5.97 ±3.4 | 4.45 ±3.45 | <0.0001* |
| Median (25th-75th percentile) | 2 (1-4) | 5 (3-8) | 4 (2-7) | |
| Range | 0-17 | 1-14 | 0-17 |
ICSI = intracytoplasmic sperm injection, IMSI = intracytoplasmic morphologically selected sperm injection, SD = standard deviation. *Mann-Whitney test.
The clinical pregnancy rate was significantly lower in ICSI as compared to IMSI (pregnancy: 34.33 vs 56.72%, respectively) (P value = 0.009) [Table 5, Figure 6].
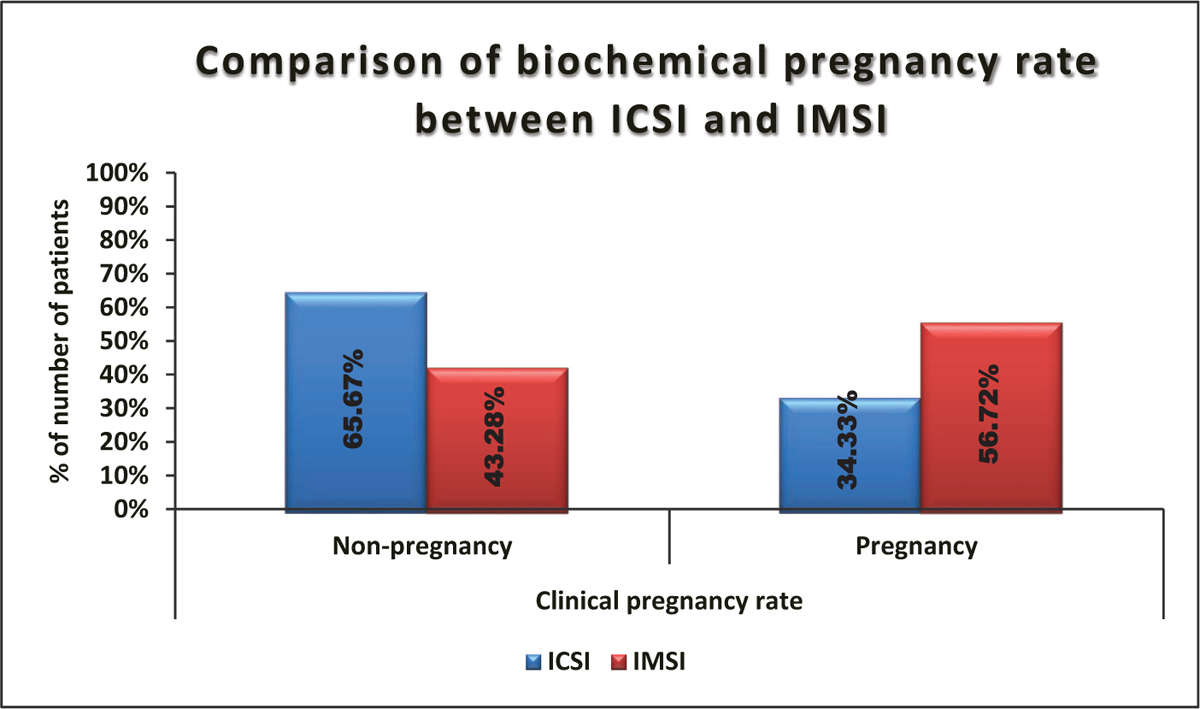
- Comparison of biochemical pregnancy rate between ICSI and IMSI. ICSI = intracytoplasmic sperm injection, IMSI = intracytoplasmic morphologically selected sperm injection
| Clinical Pregnancy Rate | ICSI (n = 67) | IMSI (n = 67) | Total | P Value |
|---|---|---|---|---|
| Nonpregnancy | 44 (65.67%) | 29 (43.28%) | 73 (54.48%) | 0.009* |
| Pregnancy | 23 (34.33%) | 38 (56.72%) | 61 (45.52%) | |
| Total | 67 (100%) | 67 (100%) | 134 (100%) |
ICSI = intracytoplasmic sperm injection, IMSI = intracytoplasmic morphologically selected sperm injection. *Chi square test.
Mean ± SD of fertilization rate in IMSI was 77.22 ± 16.83, which was significantly higher compared to ICSI (54.04 ± 16.8) (P value <0.0001) [Table 6, Figure 7].
| Fertilization Rate | ICSI (n = 67) | IMSI (n = 67) | Total | P Value |
|---|---|---|---|---|
| Mean ± SD | 54.04 ± 16.8 | 77.22 ± 16.83 | 65.63 ±20.4 | <0.0001* |
| Median (25th-75th percentile) | 53 (41-66) | 80 (66-88) | 66 (50-82.5) | |
| Range | 18-86 | 40-100 | 18-100 |
ICSI = intracytoplasmic sperm injection, IMSI = intracytoplasmic morphologically selected sperm injection, SD = standard deviation. *Independent t test.
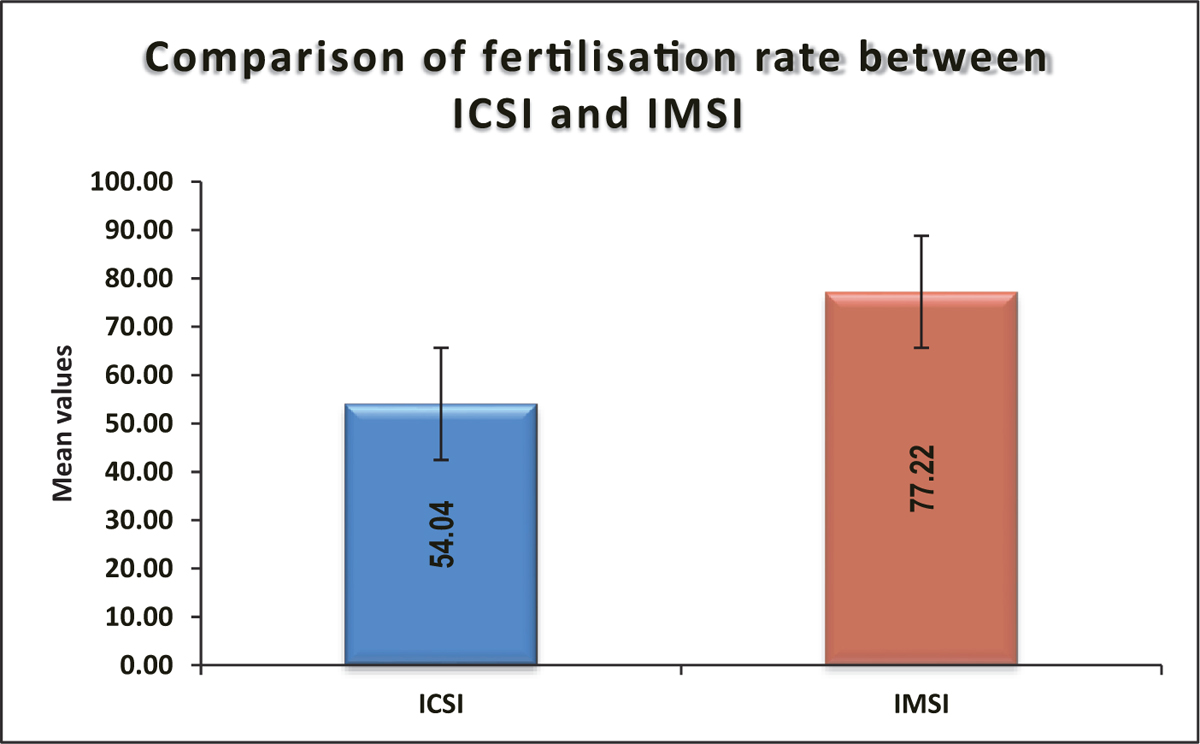
- Comparison of fertilization rate between ICSI and IMSI. ICSI = intracytoplasmic sperm injection, IMSI = intracytoplasmic morphologically selected sperm injection
Mean ± SD of % grade A in IMSI was 61.27 ± 16.02, which was significantly higher compared to ICSI (40.91 ± 21.45) (P value <0.0001) [Table 7, Figure 8].
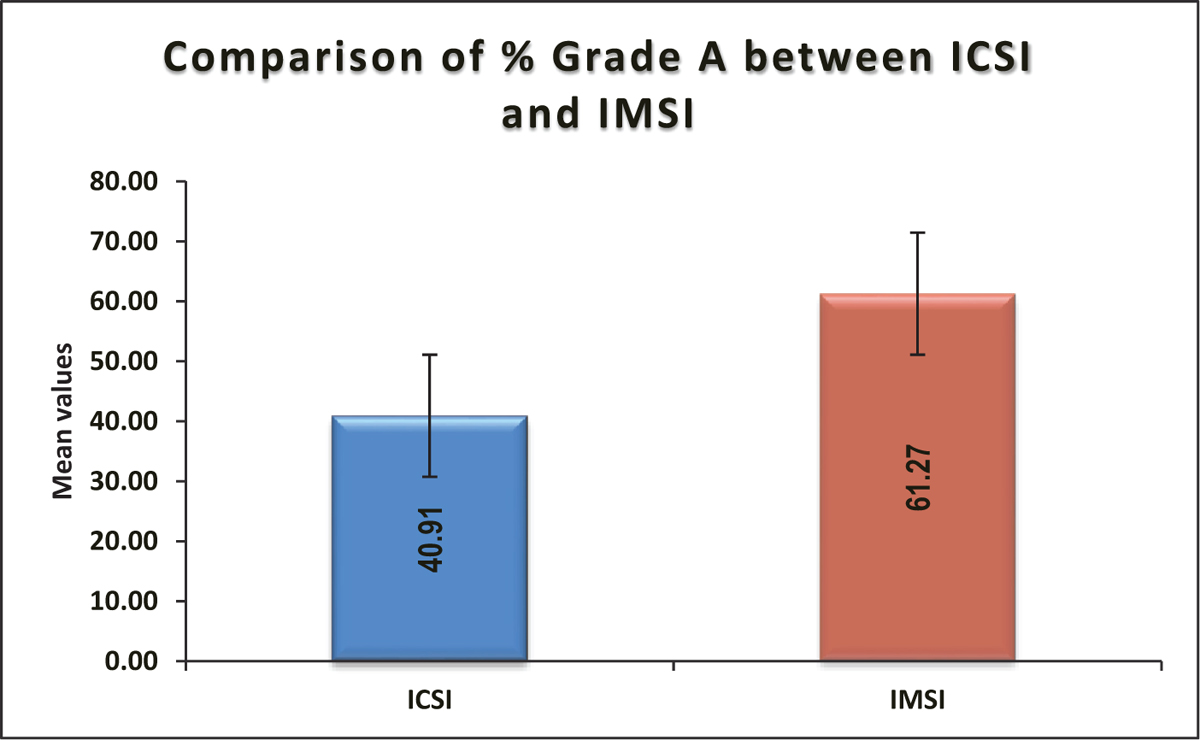
- Comparison of % grade A between ICSI and IMSI. ICSI = intracytoplasmic sperm injection, IMSI = intracytoplasmic morphologically selected sperm injection
| % Grade A | ICSI (n = 67) | IMSI (n = 67) | Total | P Value |
|---|---|---|---|---|
| Mean ± SD | 40.91 ± 21.45 | 61.27 ± 16.02 | 51.09 ± 21.45 | <0.0001* |
| Median (25th-75th percentile) | 40 (25-60) | 62 (50-70.5) | 53 (37.25-66) | |
| Range | 0-80 | 25-100 | 0-100 |
ICSI = intracytoplasmic sperm injection, IMSI = intracytoplasmic morphologically selected sperm injection, SD = standard deviation. *Independent t test.
Statistical Analysis
The presentation of categorical variables was done in the form of number and percentage (%). On the other hand, the quantitative data were presented as the means ± SD and as medians with 25th and 75th percentiles (interquartile range). The data normality was checked using the Kolmogorov–Smirnov test. In cases in which the data were not normal, we used nonparametric tests. The following statistical tests were applied for the results:
The association of the variables that were quantitative and not normally distributed in nature was analyzed using the Mann–Whitney test (for two groups) and the Kruskal–Wallis test (for more than two groups). Variables that were quantitative and normally distributed in nature were analyzed using independent t test (for two groups) and analysis of variance (ANOVA; for more than two groups).
The comparison of variables, which were qualitative in nature, was analyzed using the Chi-Square test. If any cell had an expected value of <5, then Fisher's exact test was used.
The Spearman rank correlation coefficient was used to assess the correlation between sperm morphology (%) and DNA fragmentation index (%).
The data entry was performed in the Microsoft Excel spreadsheet, and the final analysis was done using the Statistical Package for Social Sciences (SPSS) software, version 25.0 (IBM, Chicago, USA).
For statistical significance, a P value <0.05 was considered statistically significant.
DISCUSSION
The aim of this prospective study was to compare the fertilization rate, biochemical pregnancy rate, and the production of good quality embryos with respect to the micromanipulation technique (IMSI vs ICSI).
The choice of microinjection technique becomes of utmost importance when the couple has identifiable male factor infertility.
Our study included patients with semen morphology of 4% or less or a raised DNA fragmentation index (>15%) from a clinical setting where the choice of technique (ICSI/IMSI) used for treatment was made based on morphology of sperm to get the best quality embryos for transfer considering both sperm and oocyte quality.
The results showed a higher fertilization rate (77.22 vs 54.04%, P value <0.0001) and a higher quality embryo rate (61.21 vs 40.91%, P value <0.0001) in IMSI cycles compared to ICSI cycles and were statistically significant. This shows that IMSI has given better fertilization rates compared to ICSI in couples with poor sperm morphology.
The difference in biochemical pregnancy rate (56.72 vs 34.33%) between the two groups was statistically significant.
A possible explanation for this finding was the use of IMSI in the males, specifically in cases with poor morphology of sperm/teratozoospermia. IMSI has increased the fertilization rate in this group, which, in turn, was associated with good embryo quality and clinical pregnancy rate. This is because the IMSI technique allows the evaluation of detailed morphological features and subtle organelle malformations in motile spermatozoa under ultramagnification that an embryologist might consider normal for fertilization using ICSI at lower magnification, especially when the normal sperm nucleus is a significant factor for successful implantation and pregnancy in IMSI procedures. Therefore, although the number and quality of oocytes were average, obtaining the good morphology spermatozoa for treatment in severe male factor cases was seen to be beneficial in contributing to successful outcomes in the male factor group.
In reference to a case study done by Kim et al., the pregnancy and implantation rates with IMSI were significantly higher than those of the ICSI cycles (33.3 vs 12.5% and 14.6 vs 5.4%, respectively; P < 0.05).
This study shows that more clinical pregnancies are achieved with higher magnification sperm selection and support IMSI in populations with poor sperm quality.[9]
Similarly, in another study by Luna et al., the IMSI group had significantly more good quality embryos at day 3 (95 vs 73%) and higher blastocyst development rates (33 vs 19%), compared with the ICSI group (P < 0.001). Significantly higher implantation rates were observed in the IMSI group compared with the ICSI group (57 vs 27%; P < 0.05).[10]
In another reference study done by Goswami et al., the study was done to assess the outcomes of the IMSI technique compared with previous failed ICSI attempts in patients with Severe Oligoasthenoteratozopermia(SOAT)/Oligoasthenoteratozospermia(OAT)/Teratozospermia(T). The data analysis demonstrated a significant difference in the fertilization rate between IMSI and the previous ICSI attempts for these patients (52.0 vs 30.0%; P < 0.05). The embryo quality, implantation, and pregnancy rates with IMSI were also significantly higher than those of their previous ICSI cycles (56.4 vs 32%; 68.5 vs 30.2%; 62.4 vs 0.0%; P < 0.05).[11]
According to a study conducted by Sermondade et al., the quality of embryos observed by the time-lapse method and the clinical results of IMSI group were better than the ICSI group. On the other hand, among IMSI cases, the patients with OAT had a higher rate of chemical pregnancy and implantation rates. However, clinical pregnancy was higher in patients with teratozoospermia, demonstrating that cases with teratozoospermia and OAT are the preferential indications for MSOME and IMSI. Such cases showed that the IMSI method is a valuable choice for men with severe teratozoospermia at their first or second effort.[12]
However, some references contradicted the results seen in our study. The study by Oliveira et al. showed no statistically significant differences between the two groups with regard to rates of fertilization, implantation, and pregnancy per cycle. Their results suggested that IMSI does not provide a significant improvement in clinical outcomes compared to ICSI, at least in couples with repeated implantation failures after conventional ICSI.[13]
CONCLUSION
The study highlights the importance of determining sperm morphology to improve the effectiveness of assisted reproduction treatments. ICSI can be routinely used in treating patients with normal sperm morphology. Clinical IMSI can be used as a frontline treatment in males having severe male factor infertility, such as OAT, SOAT, and teratozoospermia, without having their first ICSI attempt as a failure. IMSI is an effective technique to create high-quality embryos for providing higher clinical pregnancy rates compared to conventional ICSI. It will reduce the economical and psychological burden on patients due to cycle cancellations and pregnancy failures. However, it is recommended that a diagnostic semen analysis, particularly morphological evaluation of semen samples using Kruger's strict criteria, should be assessed before opting for ICSI/IMSI procedure for treating patients with severe oligoteratozoospermia.
Also, the number of oocytes that fertilized, then cleaved to form embryos, and the number of good quality embryos (grade A) were more in the IMSI group reflecting an effect on the embryo quality.
We suggest that a proper randomized control trial in this regard is still needed before making any recommendations for the universal use of IMSI in cases of poor sperm morphology.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- International Classification of Diseases, 11th Revision (ICD-11) Geneva: WHO; 2018.
- [Google Scholar]
- National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356.
- [Google Scholar]
- A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37.
- [CrossRef] [PubMed] [Google Scholar]
- international estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506-12.
- [CrossRef] [PubMed] [Google Scholar]
- World Health Organization Towards more objectivity in diagnosis and management of male fertility. Int J Androl. 1987;7:1-53.
- [Google Scholar]
- Noninsulin‐dependent diabetes mellitus: effects on sperm morphological and functional characteristics, nuclear DNA integrity and outcome of assisted reproductive technique. Andrologia. 2012;44:490-8.
- [CrossRef] [PubMed] [Google Scholar]
- The sperm chromatin structure assay (SCSA) as prognostic factor in IVF/ICSI program. Reprod Biol. 2009;9:65-70.
- [CrossRef] [PubMed] [Google Scholar]
- The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270-83.
- [Google Scholar]
- Comparison between intracytoplasmic sperm injection and intracytoplasmic morphologically selected sperm injection in oligo-astheno-teratozoospermia patients. Clin Exp Reprod Med. 2014;41:9-14.
- [CrossRef] [PubMed] [Google Scholar]
- The IMSI procedure improves laboratory and clinical outcomes without compromising the aneuploidy rate when compared to the classical ICSI procedure. Clin Med Insights Reprod Health. 2015;9:29-37.
- [CrossRef] [PubMed] [Google Scholar]
- Can intracytoplasmic morphologically selected spermatozoa injection be used as first choice of treatment for severe male factor infertility patients? J Hum Reprod Sci. 2018;11:40-4.
- [CrossRef] [PubMed] [Google Scholar]
- Successful childbirth after intracytoplasmic morphologically selected sperm injection without assisted oocyte activation in a patient with globozoospermia. Hum Reprod. 2011;26:2944-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy outcomes in women with repeated implantation failures after intracytoplasmic morphologically selected sperm injection (IMSI) Reprod Biol Endocrinol. 2011;9:99.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy rates are higher with intracytoplasmic morphologically selected sperm injection than with conventional intracytoplasmic injection. Fertil Steril. 2003;80(6):1413-9. doi: 10.1016/j.fertnstert.2003.05.016. PMID: 14667877
- [CrossRef] [PubMed] [Google Scholar]







