Translate this page into:
IVF in hypogonadotropic hypogonadism: Challenges in management
Address for correspondence: Dr. Nihar Ranjan Bhoi, Department of Reproductive Medicine, Indira IVF Hospital Pvt Ltd, Udaipur-313001, Rajasthan, India. E-mail: drniharbhoi@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhoi NR, Nigam S, Chandra V. IVF in hypogonadotropic hypogonadism: Challenges in management. Fertil Sci Res 2023;10:170-3.
Abstract
Hypogonadotropic hypogonadism (HH) is a rare cause of infertility in females. Managing such a patient is a challenge. We present a 27-year-old lady who presented with primary infertility and had HH. She had multiple failed previous cycles with timed intercourse and one IUI. We primed her endometrium and cervical glands with estrogen–progestin for two cycles prior to starting stimulation. Stimulation was given by high-dose human menopausal gonadotropins for a longer duration. The first cycle failed, but the second cycle with similar stimulation resulted in pregnancy. A healthy baby girl was delivered at 37 weeks. The informed consent has been taken before writing the case report.
Keywords
Endometrium
hypogonadotropic hypogonadism (HH)
infertility
IUI
pregnancy
Learning points
HH is a rare cause of infertility in females.
Stimulation in HH to be done by both FSH and LH; either as separate injections or a single injection containing both the hormones.
Higher doses for longer duration are required for a favourable ovarian response.
Pre-treatment with a sequential estrogen-progestin combination for one or two cycles “primes” the endometrium and cervical glands and may result in better response to gonadotropins.
Background
According to the World Health Organization (WHO) classification system, Hypogonadotropic hypogonadism (HH) comes under the group I of ovulation disorders.[1] HH is mostly idiopathic but can be a part of various congenital syndromes.[2] HH manifests as primary or secondary amenorrhea and low or normal serum gonadotropins.[3] Idiopathic HH constitutes 1:50 000 patients with HH.[4] Patients with HH not only suffer from extreme physical, nutritional, and emotional stress but also from infertility, delayed puberty, and osteopenia.
Among the causes of female infertility, HH is one of the least common. Urinary-derived gonadotropins human menopausal gonadotropins (hMG) are used to replace the absent endogenous hormones and lead to conception in a significant number of cases.[5] However, the average treatment duration and the dose of hMG used are higher in patients with HH as compared to patients with other etiologies.[4] This is due to the requirement of priming by the “dormant” ovaries before achieving a follicular response.
We present detailed management and challenges faced by a patient presenting with HH, a rare cause of infertility.
Case presentation
A 27-year-old woman came to our clinic with primary infertility for the past 2 years. She was diagnosed with HH at 15 years of age when she was investigated for primary amenorrhea. She was on cyclical estrogen and progesterone pills for withdrawal bleeding. Prior to her presentation to our clinic, she had a few cycles of ovulation induction and timed intercourse. She also had one cycle of ovulation induction and IUI, but it failed. Her examination revealed her to be in good general condition with well-developed secondary sexual characters. Her last investigations showed low FSH (0.5 mIU/mL), low LH (0.9 mIU/mL), and serum prolactin (4 ng/mL). The MRI of the brain was normal. Her HSG was suggestive of a normal endometrial cavity and patent Fallopian tubes.
Investigations
Investigations showed the hemogram, thyroid profile, and glycemic status to be within normal limits. HIV, HBsAg, anti-HCV, and VDRL were non-reactive. Her AMH was low (1.4 ng/mL).
Her ultrasonography revealed a normal size uterus with right ovary 2 antral follicle count (AFC) and left ovary four AFC (Figures 1 and 2).
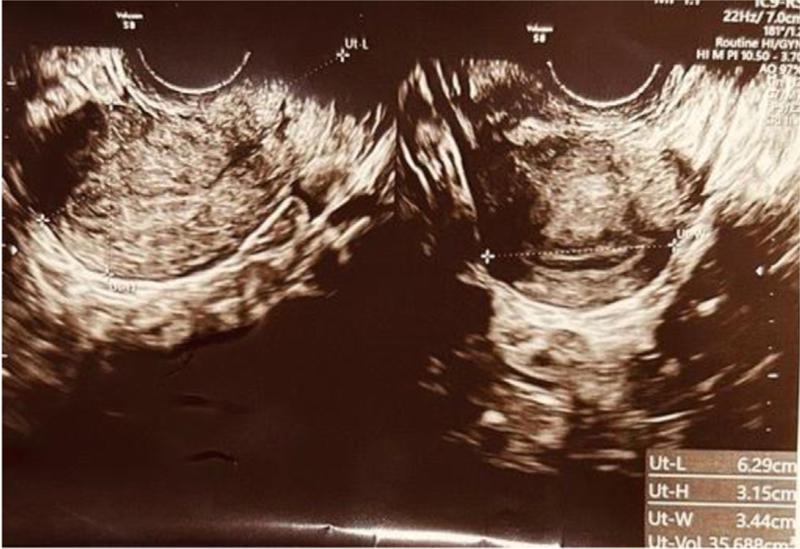
- The TVS image showing mid-sagittal and transverse section of uterus measuring 6.29 * 3.15*3.44 cm.
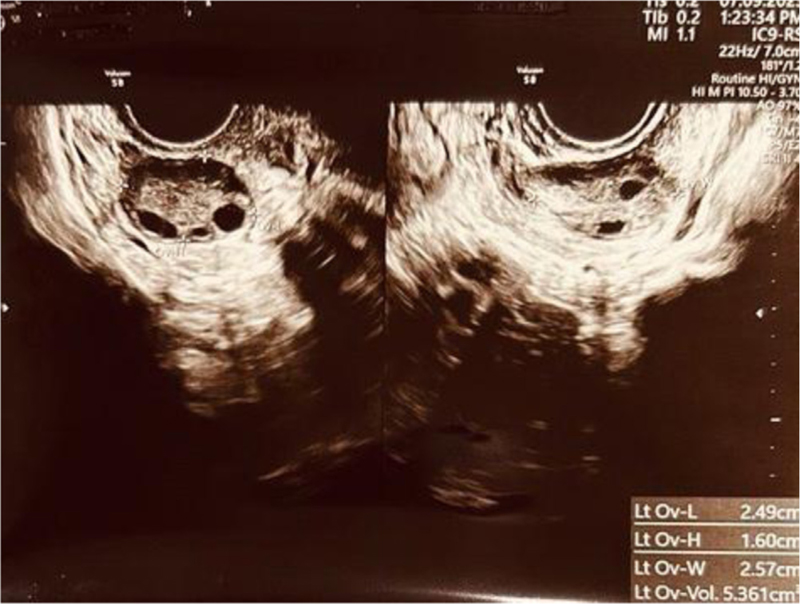
- The USG (TVS) showing left and right ovary with antral follicle count (AFC) of 6-7.
The husband's semen analysis parameters were within normal limits.
Treatment
In view of the multiple failed cycles of ovulation induction with timed intercourse and a failed IUI, she was planned for IVF. She was primed with estrogen–progestin for two cycles of withdrawal bleeding before the stimulation.
During the first treatment cycle, ovarian stimulation was started with injection hMG (Menopur, Ferring, Germany) 375 IU daily. The detailed follicular monitoring sheet for the first treatment cycle is provided in Table 1.
| Day | Right ovary Follicular size [in mm] (number of follicles) | Left ovary Follicular size [in mm] (number of follicles) | Endometrial thickness (in mm) |
|---|---|---|---|
| 1st | 2-3 (3) | 2-3 (4) | Shedding |
| 6th | 8 (1), 6 (2) | 6-7 (4) | 5 |
| 9 th | 11 (3) | 11 (2), 10-11 (2) | 6 |
| 11th | 15 (3), 10 (1) | 14 (2), 12-13 (2) | 6.6 |
| 13th | 18 (1), 17 (2), 14 (1) | 17-18 (3), 16 (1) | 6.6 |
| 14th | 19 (3), 15-16(1) | 19 (3), 18 (1) | 7 |
The same dose was continued, and the patient was spl regarding the long duration of ovarian stimulation due to HH. She was given injections of hMG (Menopur, Ferring, Germany) for 14 days. Trigger by 250 mcg recombinant human chorionic gonadotropin (hCG) (Inj. Ovitrelle, Merck Serono) was given. Ovum pick-up was done; a total of 11 oocytes were retrieved, out of which three were M2 oocytes, three were M1 oocytes, and four were germinal vesicles (GV). Intracytoplasmic sperm injection (ICSI) was done. Two blastocysts (3BB, 3AB) were formed. Her embryo transfer was done, but beta-human chorionic gonadotropin (βHCG) came negative.
After counseling, she was planned for the second treatment cycle. Injection of hMG (Menopur, Ferring, Germany) 375 IU (Menopur, Ferring) was given for 14 days. The detailed follicular monitoring sheet for the second treatment cycle is provided in Table 2.
| DAY | Right ovary Follicular size [in mm] (number of follicles) | Left ovary Follicular size [in mm] (number of follicles) | Endometrial thickness (in mm) |
|---|---|---|---|
| 1st | 2-3 (4) | 2-3 (4),2(2) | Shedding |
| 6th | 7 (3), 6 (2) | 7-8 (6) | 5 |
| 9th | 10 (4) | 12 (2), 10-11 (2),9(2) | 6.2 |
| 11th | 15 (3), 10 (1) | 14 (2), 12-13 (2) | 6.6 |
| 13th | 17 (2), 16(1), 14 (1) | 17-18 (3), 16(1), 10-11(2) | 6.6 |
| 14th | 19 (2), 17(1), 15-16 (1) | 20 (2),19 (1), 18 (1), 12 (1) | 7 |
Total 10 oocytes were retrieved out of which six were M2 oocytes. ICSI was done.
Outcomes
One Day 5, embryo (4AA) (Gardner embryo grading criteria) was formed and embryo was transferred. Tablet estradiol 2 mg three times a day, progesterone gel 8% P/V twice a day, and tablet aspirin 75 mg once a day were given as luteal phase support. Subsequently, this resulted in a clinical singleton pregnancy. Antenatal period was uneventful. She delivered a baby girl weighing 3.2 kg at 37 weeks.
DISCUSSION
Ovulatory disorders are classified into three groups by the World Health Organization (WHO). Hypothalamic-pituitary failure (HPF) leading to HH falls in Group I. Group II comprises diseases that affect the hypothalamo-pituitary-ovarian axis, and Group III comprises disorders that lead to ovarian failure. In Group I, idiopathic hypogonadotropic hypogonadism (IHH) is the most common cause of HPF and occurs due to the congenital absence of gonadotropin hormone-releasing hormone (GnRH). Acquired causes of HPF include panhypopituitarism caused by acute ischemia of the pituitary gland (pituitary apoplexy), autoimmune hypophysitis, postpartum hemorrhage, significant head trauma, and compression of the pituitary by pituitary adenomas or by brain tumors. A thorough patient history can help identify the cause and make decisions regarding the investigation to be done and treatment. Baseline investigations done are measurements of FSH, LH, and estradiol (E2). Measurement of thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), and growth hormone is required only in certain groups of patients. To rule out morphological pituitary abnormalities or tumors, MRI head is indicated. Treatment depends on the disease and the patient's needs.[6]
In female patients with HH, both FSH and LH need to be co-administered to obtain adequate follicular steroidogenesis, as both hormones are low. The FSH/LH ratio should be 2:1 in the first half of the stimulation cycle.[7,8,9] Injection hMG (Menopur, Ferring, Germany) has both FSH and LH and were hence used in our patient for stimulation. r-FSH and r-LH can both be used as separate injections. Kumback and Kahraman reported similar results with both types of stimulation in HH patients.[10] Because of the low levels of FSH and LH, downregulation by either a gonadotropin-releasing hormone agonist (GnRH a) or an antagonist (GnRH anta) is not required.
Priming of the endometrium and cervical glands with estrogen–progestin may result in a better response to stimulation.[11] We gave estrogen–progestin for two cycles prior to stimulation to the patient.
Prediction of an individual patient response to hMG (Menopur, Ferring, Germany) treatment cannot be based on baseline of LH and FSH. Only after a patient is subjected to treatment, the response assessed. Prior to consulting us, she had received hMG stimulations in previous cycles, but ovarian response was low due to the low dose of hMG used. The need for a higher dose and duration of hMG to induce ovulation in an HH female patient has already been documented.[5] However, the patient needs proper counseling before starting such a stimulation. Yilmaz et al. showed use of higher total doses of gonadotropins for longer duration in females with HH.[12] A GnRH agonist causes a natural endogenous LH surge. So, it cannot be given to HH patients due to low endogenous LH levels. hCG triggers should be used in these patients.[13]
The current case report clearly illustrates that in this specific group of patients, it is worthwhile to use higher doses of hMG for a longer duration. In female patients with HH, the threshold for ovarian response may differ substantially from that established for other patients. We conclude that patients with HH undergoing ovarian stimulation for IVF should be carefully assessed for ovarian response before giving up on obtaining mature oocytes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The reproductive performance of women with hypogonadotropic hypogonadism in an in vitro fertilization and embryo transfer program. J Assist Reprod Genet. 2005;22:1671.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroendocrine abnormalities in hypothalamic amenorrhea: spectrum, stability, and response to neurotransmitter modulation. J Clin Endocrinol Metab. 1999;84:19011.
- [CrossRef] [Google Scholar]
- Management of ovulation induction and intrauterine insemination in infertile patients with hypogondotropic hypogonadism. J Gynecol Obstet Hum Reprod. 2019;48:833-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy in women with Kallmann's syndrome. Fertil Steril. 1995;63:494-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hypothalamic-Pituitary-Ovarian Axis disorders impacting female fertility. Biomedicines. 2019;7:5. doi: 10.3390/biomedicines7010005
- [CrossRef] [PubMed] [Google Scholar]
- Recombinant LH (lutropin alfa) for the treatment of hypogonadotrophic women with profound LH deficiency: a randomized, double-blind, placebo-controlled, proof-of-efficacystudy. Clin Endocrinol. 2008;69:471-8.
- [CrossRef] [PubMed] [Google Scholar]
- The effectiveness and safety of recombinant human LH to support follicular development induced by recombinant human FSH in WHO group I anovulation: evidence from a multicentre study in Spain. Hum Reprod. 2001;16:2525-32.
- [CrossRef] [PubMed] [Google Scholar]
- Reevaluation of the roles of luteinizing hormone and follicle-stimulating hormone in the ovulatory process. Hum Reprod. 1991;6:1206-12.
- [Google Scholar]
- Women with hypogonadotropic hypogonadism: cycle characteristics and results of assisted reproductive techniques. Acta Obstet Gynecol Scand. 2006;85:1453-7.
- [CrossRef] [PubMed] [Google Scholar]
- Superovulation strategies in assisted conception. In: Rao KA, ed. Principles and Practice of Assisted Reproductive Technology. Delhi: Jaypee Publishers; 2014. p. :538-51.
- [CrossRef] [Google Scholar]
- The reproductive outcome of women with hypogonadotropic hypogonadism undergoing in vitro fertilization. Syst Biol Reprod Med. 2015;61(4):228-32.
- [CrossRef] [PubMed] [Google Scholar]
- Drugs for controlled Ovarian Stimulation. In: Rao KA, ed. Principles and Practice of Assisted Reproductive Technology. Delhi: Jaypee Publishers; 2014. p. :529-37.
- [CrossRef] [Google Scholar]







