Translate this page into:
Male infertility-evaluation and management at a glance
Address for correspondence: Dr Navdeep Kaur Ghuman, M.S.(Obstetrics and gynaecology), DNB (Obstetrics and gynaecology), FMAS, Associate Professor, Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, Jodhpur, Rajasthan, India. E-mail: drnavdeepghuman@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Poor semen quality contributes to the sub-fertility in 30%–50% of couples undergoing IVF. Ever-changing societal values and its stress, professional pressures, delayed parenthood and rampant obesity are fuelling an exponent increase in male infertility. Traditionally evaluation and management of male infertility relied heavily on semen analysis. The need for standardization of procedures for semen analysis leads to conception of WHO laboratory manual for the examination of human semen in 1980. It has been since revised and updated four times in light of emerging evidence. But time and again these reference values have been shown to be ineffective in reliably predicting the fertility status of men and has highlighted the fact that semen analysis needs to be complemented by a comprehensive history taking, physical examination, and relevant endocrine, genetic, and other investigations. In present scenario, role of IUI with husband/partner’s sperm for male infertility is limited except in couples with physical, ethical or moral objection to IVF/ICSI. ICSI has revolutionised the treatment of male infertility. Surgical sperm retrieval in the form of PESA, TESA and open testicular biopsy has allowed azoospermic patients the opportunity of biological paternity.
Keywords
ICSI
IUI
male infertility
surgical sperm retrieval
INTRODUCTION
In paucity of population based epidemiological studies the true prevalence of male sub-fertility is difficult to estimate. However, NICE guidelines quote 35% of infertility cases due to factors in male.[1] In Indian context, few hospital based studies have found a 22.4%[2] to 64.2%[3] prevalence of deranged semen parameters amongst couples attending for infertility.
The published literature does attribute a number of pathologies that are causative factors of male subfertility, and further reports that describe the treatment of several of such afflictions. Despite these a number of questions about many aspects of male infertility remain unanswered.
The second issue that confounds clinical practitioners is the diagnostic approach to a patient of male infertility. While semen analysis hold an undisputable key role in the diagnostic process it needs to be supplemented with clinical and physical examination. The advances in Endocrinological and Genetic testing and sperm function studies have added a new dimension to diagnostic work up in cases of male-infertility. The availability and economics of such tests is another issue specially in the Indian context.
This study attempts to address these issues by analysing the available literature and throw light on the aetiological aspects of male infertility. It then proceeds to appraise the published evidence and create an evidence based practical diagnostic approach to a case of male-subfertility.
Etiology
Conditions leading to male infertility can be catalogued in following atieo-physiological categories[4]:
Hypogonadotropic hypogonadism (Hypothalamic pituitary dysfunction) (1%–2%)
Obstructive causes (Sperm transport mechanism failure accounting for 10%–20%)
Primary testicular failure (30%–40%, of which about 15% to 25% are due to genetic causes)
Unexplained/Idiopathic causes (40%–50% of cases).
Diagnostic work-up
The diagnostic framework of male subfertility warrants a systematic approach to ascertain the impact of past factors on present fertility. A through medical history and physical examination followed by semen analysis and hormonal profile are the key elements of diagnostic work-up of male subfertility. More elaborate testing in form of genetic tests and sperm function tests may be necessary depending upon these initial results.
Clinical history
Male fertility has traditionally been equated with vigour and strength. Infertility, consequently can affect the self-esteem of male partner making them more reluctant to answer personal questions. While it is imperative to approach with sensitivity and due respect to privacy an effort at building rapport facilitates an elaborative history taking. A detailed medical history should include[5]:
Developmental History: Onset of puberty, history of cryptorchidism, history of anosmia/hyposmia, history of galactorrhoea
History pertaining to androgen deficiency: Ejaculation problems, loss of libido, change in shaving frequency, loss of body hair, loss of muscle mass, breast tissue development, voice change, fatigue, poor ability to concentrate
Infection history: Mumps, sexually transmitted diseases, prostatitis
Surgical History: Hernia repair, vasectomy, hydrocele surgery
Trauma/Injury to groin
General health of the men, with particular emphasis on the presence of diabetes, respiratory issues
Drugs/ Environmental − Smoking, alcohol, anabolic steroids, chemotherapy, toxic chemicals and radiation, recreational drugs (marijuana)
Sexual history: Libido, frequency of sexual intercourse, previous conceptions, previous fertility treatment
Family history: hereditary conditions
Relevant history on side of female partner
Physical examination
A thorough general physical and genital examination to look for evidence of sexually transmitted diseases, conditions of testis, epididymis and presence of hernias should be performed.
General Physical examination: The presence of male pattern escutcheon and distribution and amount of axillary hair, pubic hair and beard suggests normal endocrine status. Small testes, phallus, and prostate, scant pubic and axillary hair, disproportionately long arms and legs from delayed epiphyseal closure (arm span ≥5cm greater than height is suggestive of Klinefelter’s syndrome), reduced male musculature, gynaecomastia and persistently high-pitched voice are suggestive of hypogonadism before puberty.[6]
Testicular examination: Prader orchidometer consisting of a string of twelve numbered wooden or plastic beads of increasing size from about 1 to 25 mls should be used for testicular volume measurement.[7] Diminished testicular volume and soft consistency is suggestive of germinal tissue loss as 85% of testicular mass is constituted by germinal tissue. Although ethnic and racial origin influences testicular size, testicular growth is an indicator of pubertal progression and testicular volumes of <4ml are prepubertal, 4–15 ml are considered peri-pubertal and 12–25ml are taken as adult size testis.[8]
Scrotal examination: Scrotal examination should be conducted for any masses, scars of previous surgeries, varicocele and presence of palpable epididymis. The presence of a varicocele should be confirmed with the man standing and performing a Valsalva manoeuvre. Length of stretched penis ranges from 10–17 cm in adults and 4–8 cm in prepuberty.[9] Examination for presence of hernias and any evidence of sexually transmitted diseases should be performed.
DIAGNOSTIC MODALITIES
Semen analysis
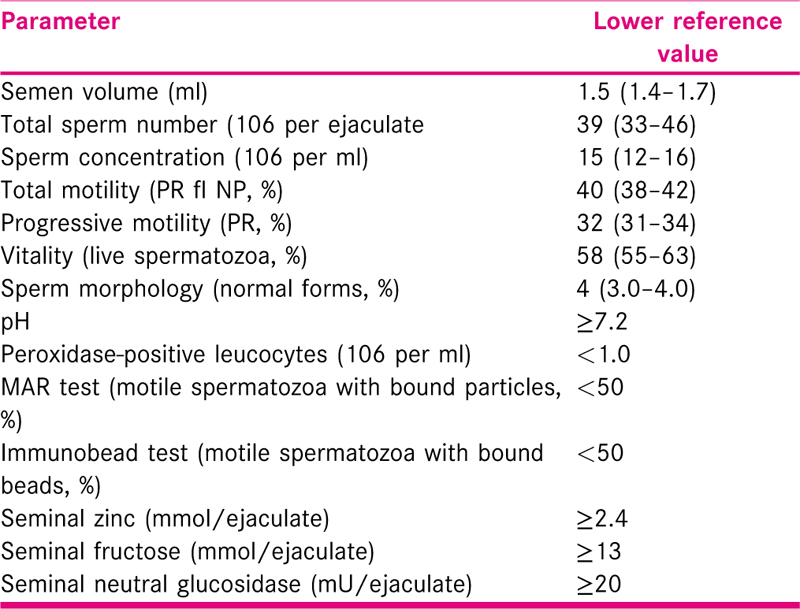
Semen analysis is often the first investigation a male partner undergoes for assessment of his fertility potential. The fifth and latest edition of WHO manual for examination of human semen was published in 2010, which offers detailed methodology for semen analysis to ensure improved comparability of results.[10] WHO recognises the need for further research to prove the benefit of automated systems including CASA for semen analysis before recommending them for clinical use. WHO prescribed reference ranges [Table 1] are based data 400–1900 semen samples from fertile men of eight countries. Studies have found WHO criteria to be sensitive tool with low specificity to detect true semen abnormality.[11] A single semen analysis has a false positive rate of 10% while two tests will falsely identify only 2% men as having abnormal semen parameters.[12] Ideally the second analysis should be scheduled at three months’ time from the first abnormal one unless this delay causes undue anxiety to male partner in which case it can be repeated in 6–8 week time. If first analysis show azoospermia, the second analysis should be performed in 2–4 weeks.[13] Among infertile males 3.9%–15.6% will show antibodies against sperms on semen analysis and the prevalence depends upon the screening method used. The WHO suggests that if more than 50% of motile sperms are bound to these antibodies, the sperm penetration in cervical mucus and in-vivo fertilisation is impaired.[14] Couples should be counselled regarding unclear consensus on the significance of these antibodies and uncertainty of the effectiveness of corticosteroids to correct this.[15]
Endocrinology profile
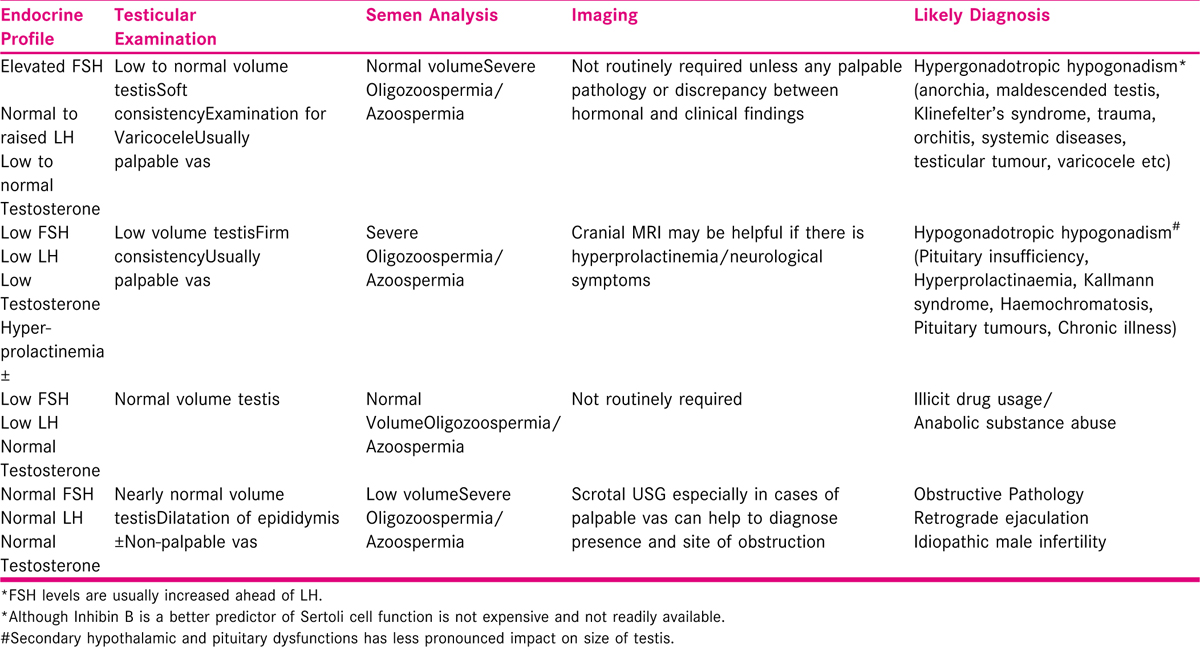
Hormone testing is the next important investigation which can provide vital inputs to help differentiating between different aetio-pathological categories [Table 2] when two semen analysis show severe oligozoospermia or azoospermia. Although the importance of FSH, LH, prolactin and testosterone testing cannot be undermined in severe male factor infertility few physiological variations have to be borne in mind while interpreting their levels. A single LH level may be erroneous owing to its pulsatility and shorter half-life. FSH on the other hand has longer half-life and more likely to accurate on single blood sample. Diurnal variations in testosterone levels are well documented and morning sample for its testing should be preferred. Furthermore, total circulatory testosterone levels are influenced by variations in sex hormone binding globulin (SHBG) and numerous factors affecting SHBG dynamics. Total circulatory testosterone may show normal levels in spite of low biologically active free testosterone in hyperthyroidism, liver disease and oestrogen excess due to increased SHBG levels in these conditions. In spite of low production of testosterone 40% of Klinefelter’s patients may be tested to have normal range testosterone levels due to increased SHBG levels following androgen excess.[16] On the contrary obesity, hypothyroidism, Type 2 diabetes and acromegaly by lowering SHBG levels lead to falsely sub-normal total testosterone levels. An estimation of free testosterone level more accurately represents biological activity but is limited by its standardisation and interpretation. Therefore, total testosterone levels warrant interpretation in light of clinical picture and FSH and LH levels. Free androgen index is particularly helpful along with total testosterone levels in clinical practice wherever there is discrepancy in testosterone levels and clinical picture.
Diagnostic imaging
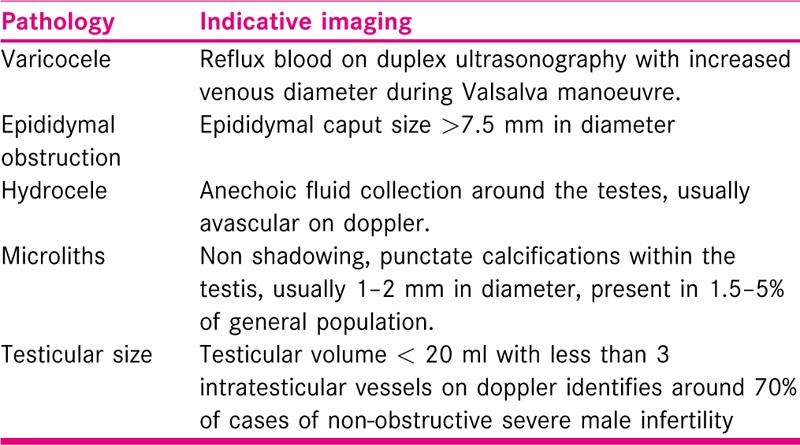
Currently, routine scrotal and testicular ultrasonography is not endorsed in male infertility except where there is inconclusive diagnosis or clinically palpable pathology. Ultrasonography has been reported to improve detection of scrotal pathologies and provides a more precise estimation of testicular volume, making it the imaging procedure of choice for male subfertility cases. Varicocele is the most common pathology detected on testicular ultrasound in these cases with hydrocele, epididymal cyst and spermatocele trailing behind.[17] Given the fact that only clinical palpable varicoceles are implicated in male infertility present guidelines do not support the use of routine ultrasonography to detect sub-clinical varicocele.[18] Trans-rectal USG is recommended in suspected cases of obstructive azoospermia with palpable vas deferens with heightened suspicion of ejaculatory duct obstruction. Although, testicular ultrasound also scores over palpation in its early detection of testicular tumours; which occur more frequently in sub-fertile males (1in 200-300 cases), this does not indicate routine ultrasonography in infertile men.[19] The 7.5–12 MHz high-resolution linear transducer is best suited for performing testicular ultrasounds. A cranial MRI is usually suggested for patients with high S. prolactin levels or with presence of visual symptoms. The diagnostic parameters indicative of scrotal pathologies are described in Table 3.
Genetic testing
Chromosomal anomalies are detected at an increased frequency of approximately 10-15-fold, in severe male infertility cases as compared to fertile males.[20] Karyotype, Y micro-deletions (Azoospermia factor- AZF deletions) and cystic fibrosis trans-membrane conductance regulator (CFTR) mutations are the fundamental genetic tests recommended if sperm concentration < 5 million/ml. Genetic testing to diagnose FSH receptor defect and other spermatozoa epigenetic are currently experimental and are not recommended in a clinical setting. Klinefelter’s syndrome affects 1 in 600 males and is most common numeric chromosomal anomaly encountered among infertile men accounting for 14% of azoospermia cases.[21] 95% men with clinically manifested cystic fibrosis are infertile and congenital bilateral absence of vas deferens (CBAVD) is most common underlying cause. AZF deletions are identified in 10%–12% and 2% of oligospermic and azoospermic men respectively. AZFc deletions accounts for 80% of these mutations. AFZa deletions though relatively less frequent but are generally associated with complete testicular germ cell atrophy. As there is potential risk of transmission of these genetic aberrations to offspring emphasis should be given to genetic counselling prior to surgical sperm retrieval in these patients.[22]
Sperm function tests
A sperm requires a wide variety of functional attributes to enable it to traverse through female genital tract and successfully fertilise the oocyte. Sperm function tests therefore address a varied spectrum of these functional characteristics including sperm motility, hyperactivation, mucus penetration, zona interaction and acrosomal reaction. Some of novel tests are based on sperm proteomics, metabolomics, mitochondrial metabolism and signalling system essential for fertilisation. These tests vary in their ability to detect these complex processes and presently evidence for their clinical use is limited. Tests intended to test sperm DNA integrity using TUNEL, Comet or flow chromatin analysis likewise have limited utility in clinical practice at present owing to their non-harmonious results.[23] The tests having considerable future potential but needing validation and approval for routine clinical practice are:
Cap-score assay
Tests of sperm capacitation
Tests of hemizona and zona pellucida binding
Sperm penetration assay or sperm capacitation index or zona-free hamster oocyte penetration assay
CatSper expression testing
Assessment of ROS
Sperm proteomics
Sperm mitochondrial function tests
Sperm viability testing
Testicular biopsy
Although diagnostic testicular biopsies in infertile males are not recommended, a biopsy taken in setting of surgical sperm retrieval sent for histopathological examination to evaluate spermatogenic capacity of testis.[24]
MANAGEMENT OF MALE INFERTILITY
Lifestyle measures
The effect of lifestyle factors on general well-being and fertility is well-documented.[25] These lifestyle factors include non-modifiable elements like age and certain modifiable choices like body weight, smoking, stress, caffeine, and alcohol consumption. Although, deterioration of semen parameters especially semen volume and motility are widely accepted by most researchers, the evidence of negative effect of advancing paternal age on reproduction is largely inconclusive especially up to 45 years of age.[26] Robust evidence for association of structural congenital malformations with progressing paternal age is lacking though some neuro-cognitive disorders like schizophrenia, autism and bipolar disorders have been linked to advanced paternal age especially after 50s.[27]
Effect of obesity on male infertility is multipronged. Obesity is linked to deterioration of semen quality and lowered testosterone levels[28] and males having a BMI of over 30 are likely to take longer to conceive.[29] Similarly, physically active men who exercised 3-4 hours a week were found to have improved semen scores over men who did not involve in physical activity.[30] A diet rich in carbohydrates, fibre, folate, lycopene with lower amounts of fats as well as consuming vegetables and fruits were associated with improved semen parameters in men.[31] Cochrane group demonstrated that a low-quality evidence exits showing anti-oxidant intake may lead to improved live birth rate, although this was based on only 44 births from 4 small studies.[32] However, recent randomised study with reasonably decent participants with male factor infertility (85 in antioxidant group and 85 in placebo group) has refuted beneficial effect of anti-oxidants on semen parameters or pregnancy occurrence.[33] Vaginal intercourse every 2-3 days maximise odds of natural conception and should be recommended especially as timed intercourse may be emotionally stressful.[29] Stress can be a contributing factor to male subfertility[34] and early recognition and providing psycho-therapeutic options is of paramount importance. Evidence is inconsistent regarding the beneficial effect of acupuncture on male fertility.
Weak evidence linking smoking to negatively impact male fertility exists and it is advisable to encourage men to quit smoking for general well-being.[35] Likewise, excessive alcohol is considered detrimental to semen quality but 3 to 4 units per day are unlikely to affect their semen quality. No association between caffeinated beverages and male sub-fertility has been evidence proved. Elevated scrotal temperature has been associated with poor semen parameters but role of wearing loose-fitting underwear to improve fertility is controversial.[1] Illicit drug usage, environmental pollutants, occupational exposures and radiation exposure may influence male fertility negatively.[36]
Medical treatment
Hypogonadotropic hypogonadic men with exception of cryptorchidism can be effectively treated with gonadotrophins. The medical therapy involves normalisation of intra-testicular testosterone by use of Human chorionic gonadotrophin (hCG) or Pulsatile gonadotrophin-releasing hormone (GnRH) for 3-6 months following which a combination of FSH and LH is employed to initiate spermatogenesis.[4,15] Studies have shown normalization of testicular function in 80% of cases with 50%–70% pregnancy rates. A high prolactin level suggestive of a possible micro or macro-adenoma may be another treatable cause of male sub-fertility responding to bromocriptine or cabergoline.
Gonadotrophins do not confer benefit in unexplained male sub-fertility. Similarly, the use of selective oestrogen receptor modulators (clomiphene citrate and tamoxifen), kinin enhancing drugs or bromocriptine is not recommended in setting of unexplained subfertility due to lack of evidence.
Alpha agonist drugs by increasing bladder sympathetic tone and anti-cholinergic agents by inhibiting para-sympathetic stimulation is often used in cases of retrograde ejaculation. Imipramine is the most commonly used drug for this indication. As second line of management, penile electro-vibration and recovery of sperms from buffered urine can be attempted. Overall success rate of medical therapy for retrograde ejaculation is nearly 50%, but for anejaculation the success rate of medical therapy is disappointing.[37] Penile electro-vibration by initiating reflex spinal cord activity and transrectal electro-ejaculation by stimulating nerves responsible for ejaculation may be used in an-ejaculatory patients to retrieve sperms.[4,38] Surgical sperm retrieval is the final resort if these modalities fail. Anxiolytic drugs and/or sildenafil can be helpful men with ejaculatory dysfunction with underlying psychogenic disorders.
Leukocytospermia (leukocytes>1 × 106/ml), has been proposed to cause poor semen quality. There is a divided opinion among researches regarding the need for antibiotic treatment for leucocytospermia and its role in improving semen parameters and fertility outcomes. It would be however worthwhile to send semen for culture and sensitivity testing and treat accordingly particularly if it is associated with identified infection.[39] NICE 2013 guidelines advises against the use of antibiotics in men with leucocytes in their semen unless there is an identified infection as it has not been shown to improve pregnancy rates.[15]
Alpha blockers like bunazosin and mast cell blockers have seen to improve semen parameters but need further evidence before they can be considered as therapeutic options.
Surgical options for azoospermia
Reconstructive surgery
Corrective surgical procedures including vasovasostomy and vasoepididymostomy are advocated over surgical sperm retrieval for vasectomy reversal in terms of better take home baby rates and considerably lesser cost per delivery analysis.[40] Vasectomy reversal results are influenced by the vasectomy site, interval between vasectomy and reversal and surgeon expertise. 97% patency rates and 75% pregnancy rates had been estimated following reversal within 3 years while the corresponding figures drop to 80% and 55% after 3-8 years of vasectomy.[4] It is worthwhile to consider assisted reproduction in couples who fail to conceive 12-18 months following vasectomy reversal.
The results of corrective surgeries in cases of obstruction secondary to infection are not very promising and cryopreservation of sperms for ART recovered in setting of such a surgery is always recommended to alleviate the need of future surgical procedures.[41] SSR and ICSI treatment is a good alternative in infective obstructions especially where large segment of ejaculatory system is occluded.[42] Similarly, reconstructive surgery in congenital bilateral absence of vas deferens (CABVD) is not indicated due to lack of development of major portion of vas deferens and SSR is the only viable option for this condition.[43]
Surgical sperm retrieval (SSR)
Surgical sperm retrieval is an umbrella term encompassing a number of surgical techniques aimed to provide sperms for ICSI in azoospermic men thus offering these men the prospect of biological paternity.[44] While, in obstructive azoospermia recovery of sperms from epididymis is successful in majority of cases, sperms usually need to be retrieved from testis directly in non-obstructive and idiopathic azoospermia. It may involve aspiration of sperms from testis (TESA), testicular sperm extraction (TESE) or testicular biopsy.[45] Comparatively, recently fine needle aspiration of sperms from testis (TEFNA) has been proposed as lesser invasive and better endured procedure.[46] Published literature quote 95-100% successful retrieval of sperms by percutaneous procedures in patients with carefully diagnosed obstructive azoospermia[47,48] whereas in non-obstructive azoospermia the figures stagger around 45%–55%.[48,49] In Klinefelter syndrome the success rates depend upon male age and can go up to 50% in younger males.[16,50] Histological studies in cases of non-obstructive azoospermia had found Sertoli cell only syndrome in two-thirds of these patients followed by maturational arrest and hypo-spermatogenesis.[48] A fresh approach where SSR may be planned along with oocyte retrieval or a frozen approach which involves scheduling of SSR before ICSI treatment with aim to cryo-preserve sperms obtained to be used at later date are generally accepted lines of management. For improved synchronisation of treatment and to avoid disappointments, frozen approach is preferred by most experts particularly in non-obstructive azoospermia. There is always a risk of need for repeat procedure if no or very few viable sperms survive post-thaw, which can be the case if limited number of sperms had been retrieved in initial SSR procedure and couple needs to be counselled about this scenario. Reassuringly, similar fertilisation and pregnancy rates with fresh and frozen sperms had been proposed by clinical studies.[51]
Comparative evaluation of different SSR techniques
Obstruction to the flow of semen causes dilated epididymal tubules which may be aspirated by a blind puncture of these tubules using a 21-23 gauze needle passed through scrotal skin following stabilisation of epididymis between surgeon’s thumb and forefinger.[52] This technique is relatively simpler and can be done under local anaesthesia as compared to aspiration of epididymal tubules under direct vision using microsurgical principles (MESA). The motile sperms obtained by epididymal aspiration has been found to exhibit sperm DNA damage rates comparable to that of donor sperms tested by TUNEL assay.[42] Testicular sperm aspiration (TESA) may be undertaken in the event of failure to retrieve sperms from epididymis, using a 16-18 gauze needle passed through taut scrotal skin in relatively avascular anteromedial or anterolateral testicular lower pole once testis has been stabilised. Different sections of testis may be sampled through same puncture in tunica albuginea before completely withdrawing the needle. The testicular tubules thus obtained require mechanical disruption or enzymatic treatment to liberate sperms and final results are often available only after few hours of the procedure. Evidence to support diagnostic TESA with sole purpose of identifying the presence of testicular spermatogenesis in cases of non-obstructive azoospermia is lacking.
In increasing order of invasiveness, another important technique is the extraction of seminiferous tubules from testis (TESE) through a midline or transverse scrotal incision in an adequately anesthetised male. Delivery of testicles through scrotal incision is not required and a small anterior incision in avascular plane is given on tunica albuginea and extrusion of seminiferous tubules is aided by a gentle pressure on the testicle.[52] The procedure has advantages over other techniques in terms of recovering reasonably substantial number of sperms and acquiring sample for histo-pathological examination. Performing this procedure under 10-15-fold magnification (micro-TESE) using an optical microscope or a magnifying loop is theoretically likely to increase retrieval of sperms by selecting larger opaque tubules with sperm production at the cost of increased operative time and surgical expertise required.[49]
Similar fertilisation and live birth rates were observed between sperms recovered from epididymis and testis.[51] Furthermore, there is not sufficient evidence currently to endorse one SSR technique over other[45] and underlying cause, physician preference and degree of invasiveness are the determinants of the procedure chosen.[44] Customarily more invasive procedures are approached if lesser invasive techniques fail to retrieve sperms.
Surgery for specific conditions associated with male subfertility
Incidence of undescended testis or cryptorchidism can be up to 21% in premature male neonates dropping to 2-4% of term new-born boys.[53] In majority of cases it is unilateral while in 10% cases both testes are involved. By 3 months of age half of the cases of cryptorchidism resolve spontaneously and natural descend is unlikely beyond 1 year. The condition is multifactorial with faulty anti-mullerian hormone regulation, defective androgen production and genetic factors implicated as the causes. Long-term complications of undescended testes include infertility, risk of testicular malignancy and psychological consequences. Early orchidopexy performed between 6-12 months of can lead to normal sperm count in 76% of cases as opposed to 26% if surgical correction is delayed beyond 3 years[53] in addition to reducing the incidence of malignancy.[54] Testicular atrophy due to vascular damage is a serious complication of orchidopexy. Hormonal treatment with human chorionic gonadotrophin (hCG), LH or GnRH may help in testicular descent in 20% cases.[55]
Adverse effects of dilated veins of pampiniform plexus or varicoceles on semen parameters are widely accepted but improvement in semen parameters or reproductive outcomes following varicocelectomy are controversial. Presently varicocele surgery with sole purpose of improving male fertility without any pain complains or men with subclinical varicocele is not advocated.[56]
Assisted reproduction techniques (ART)
Once used widely as first line management option for male factor sub-fertility, IUI has fallen out of favour for male infertility in light of recent evidence.[15] First introduced in early 1990s, the technique of Intracytoplasmic sperm injection (ICSI) has proved a dramatic breakthrough treatment modality for male factor subfertility. Successful fertilisation and pregnancy had been reported with injection of immature sperm cells like round spermatids and elongated spermatids.[57] The pregnancy rates observed with round spermatids markedly lower as compared with elongated spermatids and spermatozoa.[58] Also, it is imperative to advise the couple regarding possible increase of genetic and epigenetic alterations resulting from use of immature spermatids specifically when round spermatids are used. A small but statistically significant increased risk of certain genetic and developmental defects observed in off springs born from ICSI treatment should also be discussed with couples. Research is going on to devise methods/algorithms to improve sperm selection for ICSI.
Selection of sperms for ICSI treatment
Several anecdotal sperm selection tests including sperm tail flexibility test (STFT), response to hypo-osmotic solution and laser shot method had been employed to select viable and intact sperms from immotile sperm population for injection. None of these selection tests were proved to improve reproductive outcomes and did not find their place in clinical practice.[59,60]
Magnified study of sperm morphology (Intracytoplasmic morphologically selected sperm injection-IMSI) under 6000-fold magnification in comparison to 400-fold magnification used for conventional ICSI enables recognition of subtle defects in sperm organelles such as the acrosome, mitochondria, especially that of the nucleus allowing selection of highest quality sperm for ICSI. Although this selection had exhibited increased fertilisation rate and reduction in miscarriage rate strong evidence for its universal use is lacking.[61]
Between year 2005 and 2015 numerous research works focused on selection of non-apoptotic cells using approach combining density gradient centrifugation and annexin V-magnetic activated cell sorter (MACS). Apoptotic sperms with exposed phospholipid phosphatidylserine (PS) on their plasma membrane conjugate with annexin V and are retained in the cell sorter columns while non-apoptotic cells pass through under magnetic field. These studies have observed inconsistent results, with few detecting improved fertilisation rates but no improvement in clinical pregnancy or live birth rates over conventional selection.[62,63]
Chan and colleagues utilised the static surface electric charge called zeta potential possessed by mature sperms to select and separate sperms with high DNA integrity using electrostatic surface and showed improved fertilisation rates.[64] Likewise, the net negative charge on healthy sperms was employed for electrophoretic separation by others. Further, the presence of hyaluronic acid in the cumulus oophorus surrounding ovulated oocyte is exploited in commercially available dishes to select good quality sperms for ICSI based on their avidity for hyaluronic acid. This selection technique also known as Physiological Intracytoplasmic sperm injection (PICSI) was shown to result in embryo quality and development[65] without a significantly proved improvement in reproductive outcomes.[66]
Presently, robust evidence is lacking for utilisation of these selection techniques in clinical setting as most of trials have small numbers, inconsistent results and lack data on important clinical outcomes.
Augments to ICSI
Fertilisation failure is seen in 2-5% of ICSI cycles.[67] It has been proposed that during early phase of fertilisation procedure sperm specific protein is introduced in oocyte which in turn leads to oocyte activation by generating intracellular Ca+2 oscillations in oocyte. Extrapolating this observation, calcium ionophores had been employed in research settings to artificially facilitate oocyte activation in PLC zeta deficit sperms to bring about improved fertilisation.[68]Theophylline or pentoxifylline treated sperms to improve sperm motility were reported to result in improvement in fertilisation rates, implantation and clinical pregnancy rates in asthenozoospermic patients.[69,70] Further prospective studies are required for safety profile of these compounds as controversial results regarding embryotoxicity were derived from initial animal studies.
Donor sperm treatment
The decision to use donor sperm can a difficult and trying decision for a couple. Counselling should be arranged for both partners to discuss their feelings and the potential implications of using donor sperm.[71] Treatment with donor sperms is a potential option in azoospermic men where corrective surgery or surgical sperm retrieval have failed or in cases of severe azoospermia with recurrent fertilisation failure or poor embryo development. This option may be considered in selected cases severe sexual or ejaculatory dysfunction refractory to medical treatment. Use of donated sperms may have a role when risk of inheriting genetic anomaly through male gamete is significant. In developing countries few couples with male factor infertility may resort to donor sperm treatment because of financial implications of assisted reproduction.
Sperm donors are rigorously screened for sexually transmitted diseases and genetic conditions to safeguard the recipient and potential offspring resulting from sperm donation from infectious diseases and serious inheritable disorders.[72] The semen sample is quarantined for a minimum period of 6 months if serology alone has been used for testing or at least for 3 months if nucleic acid amplification testing has been performed along with serological testing, before using the sperms for any treatment.[73]
Other options
Some couples affected by irreversible male infertility may consider adopting a child.[74] Assisted reproduction units should have appointed healthcare provider or social worker trained to help and assist these couples who decide to pursue this option. Some couples may want to stop all fertility treatment and may decide to remain childless.
Future perspective
Considerable work is being ongoing to derive male germ cells from embryonic pluripotent stem cells. Stem cells are considered as potentially new therapeutic agents for the treatment of male infertility.[75] Scientific fraternity is though concerned by the genetic aberrations or epigenetic changes it might induce in offspring.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Epidemiologic and etiologic aspects of primary infertility in the Kashmir region of India. Fertility and Sterility. 1997;68:637-43.
- [Google Scholar]
- Prevalence of male factor infertility in last ten years at a rural tertiary care centre of central India: a retrospective analysis. Indian Journal of Obstetrics and Gynaecology Research. 2015;2:132-6.
- [Google Scholar]
- Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins; 2005. p. :1400.
- What every gynecologist should know about male infertility: an update. Archives of Gynecology and Obstetrics. 2012;286:217-29.
- [Google Scholar]
- Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism—pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2015;11:547-64.
- [Google Scholar]
- Measurement of pediatric testicular volume with Prader orchidometer: comparison of different hands. Pediatric Surgery International. 2005;21:517-20.
- [Google Scholar]
- Adams GR, Berzonsky M, eds. Blackwell handbook of adolescence. John Wiley & Sons; 2008.
- Growth and maturation in the male genitalia from birth to adolescence II. Change of penile length. Pediatrics International. 1987;29:220-3.
- [Google Scholar]
- World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231-45.
- [Google Scholar]
- Normal reference ranges for semen quality and their relations to fecundity. Asian Journal of Andrology. 2010;12:95.
- [Google Scholar]
- National Collaborating Centre for Women’s and Children’s Health (UK. In: Fertility: assessment and treatment for people with fertility problems. RCOG press; 2004.
- [Google Scholar]
- Epididymal more than testicular abnormalities are associated with the occurrence of antisperm antibodies as evaluated by the MAR test. Human Reproduction. 2018;33:1417-29.
- [Google Scholar]
- Fertility problems: Assessment and treatment [CG156].
- Role of sonography imaging in male infertility. International Journal of Medical and Biomedical Studies. 2019;3:101.
- [Google Scholar]
- Clinical Consultation Guide on Imaging in Male Infertility and Sexual dysfunction. European Urology Focus. 2018;4:338-47.
- [Google Scholar]
- High-resolution ultrasonography in the diagnosis of scrotal pathology: II. Tumors. J Clin Ultrasound. 1993;21:375-86.
- [Google Scholar]
- Prevalence of chromosomal abnormalities in 2078 infertile couples referred for assisted reproductive techniques. Hum Reprod. 2005;20:437-42.
- [Google Scholar]
- Sperm retrieval in adolescents and young adults with Klinefelter syndrome: a prospective, pilot study. The Journal of Pediatrics. 2016;170:260-5.
- [Google Scholar]
- A decade of experience emphasizes that testing for Y microdeletions is essential in American men with azoospermia and severe oligozoospermia. Fertility and Sterility. 2010;94:1753-6.
- [Google Scholar]
- Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Translational Andrology and Urology. 2016;5:935.
- [Google Scholar]
- Histopathological evaluation of testicular biopsy. Endocrinology of the Testis and Male Reproduction 2017:623-42.
- [Google Scholar]
- CDC Recommendations to Improve Preconception Health and Health Care—U55nited States, 2006 A Report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm
- Does age of the sperm donor influence live birth outcome in assisted reproduction? Human Reproduction. 2016;31:582-90.
- [Google Scholar]
- The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Human Reproduction. 2014;29:193-200.
- [Google Scholar]
- Influence of increasing body mass index on semen and reproductive hormonal parameters in a multi-institutional cohort of subfertile men. Fertility and Sterility. 2016;106:1070-5.
- [Google Scholar]
- Physical activity and semen quality among men attending an infertility clinic. Fertil Steril. 2011;95:1025-30. 93
- [Google Scholar]
- New evidence of the influence of exogenous and endogenous factors on sperm count in man. Eur J Obstet Gynecol Reprod Biol. 2003;110:49-54.
- [Google Scholar]
- Antioxidants for male subfertility. Cochrane Database of Systematic Reviews (Online). 2011;1:CD007411. doi:10:1002/14651858.
- [Google Scholar]
- The effect of antioxidants on male factor infertility: the Males, Antioxidants, and Infertility (MOXI) randomized clinical trial. Fertility and Sterility 2020:96.
- [Google Scholar]
- Association between socio-psycho-behavioral factors and male semen quality: Systematic review and meta-analyses. Fertil Steril. 2011;95:116-23.
- [Google Scholar]
- Cigarette smoke extract immobilizes human spermatozoa and induces sperm apoptosis. Reprod Biomed Online. 2009;19:564-571.
- [Google Scholar]
- Lifestyle factors and reproductive health: taking control of your fertility. Reproductive Biology and Endocrinology. 2013;11:66.
- [Google Scholar]
- Development and in vitro testing of a new method of urine preparation for retrograde ejaculation; the Liverpool solution. Fertility and Sterility. 2008;89:885-91.
- [Google Scholar]
- Doctor Chris G. McMahon: epidemiology and diagnosis of ejaculatory dysfunction. Transl Androl Urol. 2016;5:160-1.
- [Google Scholar]
- Treatment of leukocytospermia in male infertility: a systematic review. The World Journal of Men’s Health. 2016;34:165-72.
- [Google Scholar]
- Microsurgical vasovasostomy versus microsurgical epididymal sperm aspiration/testicular extraction of sperm combined with intracytoplasmic sperm injection. European Urology. 2000;37:609-14.
- [Google Scholar]
- Microsurgical vasoepididymostomy with sperm cryopreservation for future assisted reproduction. Int J Urol. 2000;7:435-9.
- [Google Scholar]
- Update on surgical sperm recovery-the European view. Human Fertility. 2010;1:242-6.
- [Google Scholar]
- Microdissection testicular sperm extraction. Translational Andrology and Urology. 2017;6:745.
- [Google Scholar]
- Surgical recovery of sperm in non-obstructive azoospermia. Asian J Androl. 2012;14:109.
- [Google Scholar]
- Techniques for surgical retrieval of sperm prior to ICSI for azoospermia. Cochrane Database of Systematic Reviews 2006
- [Google Scholar]
- Testicular fine needle aspiration: the alternative method for sperm retrieval in non-obstructive azoospermia. Human Reproduction. 1999;14:1785-90.
- [Google Scholar]
- Percutaneous Sperm Retrieval in Secondary Azoospermia. Urologia Internationalis. 2008;81:252-5.
- [Google Scholar]
- Testicular histology may predict the successful sperm retrieval in patients with non-obstructive azoospermia undergoing conventional TESE: a diagnostic accuracy study. Journal of Assisted Reproduction and Genetics. 2017;34:149-54.
- [Google Scholar]
- Conventional testicular sperm extraction (TESE) and non-obstructive azoospermia: is there still a chance in the era of microdissection TESE? Results from a single non-academic community hospital. Andrology. 2016;4:425-9.
- [Google Scholar]
- Reproductive outcomes in patients with male infertility because of Klinefelter’s syndrome, Kartagener’s syndrome, round-head sperm, dysplasia fibrous sheath, and ‘stump’ tail sperm: an updated literature review. Curr Opin Obstet Gynecol. 2013;25:229.
- [Google Scholar]
- Use of surgical sperm retrieval in azoospermic men: a meta-analysis. Fertility and Sterility. 2004;82:691-701.
- [Google Scholar]
- A step-by-step guide to office-based sperm retrieval for obstructive azoospermia. Translational Andrology and Urology. 2017;6:730.
- [Google Scholar]
- Cryptorchidism: classification, prevalence and long-term consequences. Acta Paediatr. 2007;96:611-6.
- [Google Scholar]
- The impact of age at orchiopexy on testicular cancer outcomes. World Journal of Urology 2019:1-6.
- [Google Scholar]
- Assessment of efficacy of varicocele repair for male subfertility: a systematic review. Lancet. 2003;361:1849-52.
- [Google Scholar]
- Intracytoplas- mic injection of spermatids retrieved from testicular tissue: influence of testicular pathology, type of selected spermatids and oocyte activation. Hum Reprod. 1997;12:1203-13.
- [Google Scholar]
- Multiple pregnancies obtained by testicular spermatid injection in combination with in- tracytoplasmic sperm injection. Hum Reprod. 1998;13:104-10.
- [Google Scholar]
- The majority of males with subnormal hypoosmotic test scores have normal vitality. Clin Exp Obstet Gynecol. 2012;39:25-26.
- [Google Scholar]
- Selection of viable spermatozoa from testicular biopsies: a comparative study between pentoxifylline and hypoosmotic swelling test. Fertility and Sterility. 2011;95:631-4.
- [Google Scholar]
- Intracytoplasmic morphologically selected sperm injection (IMSI) does not improve outcome in patients with two successive IVF-ICSI failures. Journal of Assisted Reproduction and Genetics. 2016;33:349-55.
- [Google Scholar]
- Clinical outcome of magnetic activated cell sorting of non-apoptotic spermatozoa before density gradient centrifugation for assisted reproduction. Journal of Assisted Reproduction and Genetics. 2008;25:375-81.
- [Google Scholar]
- Elimination of apoptotic spermatozoa by magnetic-activated cell sorting improves the fertilization rate of couples treated with ICSI procedure. Andrology. 2013;1:845-9.
- [Google Scholar]
- A simple zeta method for sperm selection based on membrane charge. Fertility and Sterility. 2006;85:481-6.
- [Google Scholar]
- “Physiologic ICSI”: hyaluronic acid (HA) favors selection of spermatozoa without DNA fragmentation and with normal nucleus, resulting in improvement of embryo quality. Fertility and Sterility. 2010;93:598-604.
- [Google Scholar]
- Embryo quality is improved by PICSI sperm selection. a randomized controlled trial. Fertility and Sterility. 2016;106:e307.
- [Google Scholar]
- Successful pregnancy following oocyte activation by strontium in normozoospermic patients of unexplained infertility with fertilisation failures during previous intracytoplasmic sperm injection treatment. Reproduction, Fertility and Development. 2010;22:852-5.
- [Google Scholar]
- Does the use of calcium ionophore during artificial oocyte activation demonstrate an effect on pregnancy rate? A meta-analysis. Fertility and Sterility. 2017;108:468-82.
- [Google Scholar]
- Pharmacological stimulation of sperm motility in frozen and thawed testicular sperm using the dimethylxanthine theophylline. Fertility and Sterility. 2011;96:1331-6.
- [Google Scholar]
- Selection of viable spermatozoa from testicular biopsies: a comparative study between pentoxifylline and hypoosmotic swelling test. Fertility and Sterility. 2011;95:631-4.
- [Google Scholar]
- Gamete donors’ and recipients’ evaluation of donor counselling: a prospective longitudinal cohort study. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2008;48:601-6.
- [Google Scholar]
- UK guidelines for the medical and laboratory procurement and use of sperm, oocyte and embryo donors(2019) Human Fertility. 2019;5:1-3.
- [Google Scholar]
- guidance on tests for Sexually Transmitted Infections. BASHH Clinical Effectiveness Group; 2015.
- Emotion regulation processes in couples with infertility, fertile couples and couples applying for adoption. InACBS Annual World Conference
- Human induced pluripotent stem cells and male infertility: an overview of current progress and perspectives. Human Reproduction. 2018;33:188-95.
- [Google Scholar]







