Translate this page into:
Uterine transplant
Address for correspondence: Dr. Neeti Chhabra, R-733, Second Floor, New Rajinder Nagar, New Delhi - 110 060, India. E-mail: justneeti23@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Despite the tremendous advancements made, absolute uterine factor infertility (absence of the uterus or the presence of a nonfunctional uterus) is one of the few spheres where still there is a lot of scope for research and growth. One option for this group of patients is a uterine transplant. Uterine transplant is a type of an ephemeral/quality of life-enhancing transplant. This article reviews the beginning, research in animals, application in humans, donor and recipient selection, technical details, and finally the ethical aspects of its application in the Indian context.
Keywords
Donor
ethical aspects
immunosuppression
procedure
recipient
uterine transplant
INTRODUCTION
The recent advances in the field of infertility have brought joy to many childless couples. These achievements have been extended to so many subsets of infertility treatment. Despite the tremendous advancements made, absolute uterine factor infertility (absence of the uterus or the presence of a nonfunctional uterus) is one of the few spheres where still there is a lot of scope for research and growth. Present options for women with absolute uterine factor infertility are either gestational surrogacy or adoption. With stringent rules of surrogacy involving ethical, legal, and religious constraints in various parts of the world including India and adoption being a long, tedious, and emotionally challenging option, the need was felt to think out of the box and provide these patients with a ray of hope. The uterine transplant was brought out as one such option. Cooperation between the gynecologists and transplant surgeons is needed to make this a success. In the present review, we bring out the progress made in the field so far and also address the ethical considerations for the same.
REVIEW OF LITERATURE
Uterine transplant is a type of nonvital/quality of life enhancing/ephemeral transplant. Initial research on the subject was done in animals and later on extended to humans. The following Table 1 summarizes the experiments in animals.[1]
The first uterine transplant was performed on April 6, 2000.[2] The recipient was a 26-year-old female who underwent peripartum hysterectomy 6 years earlier due to postpartum hemorrhage. The donor was a 46-year-old lady who had multiloculated ovarian cysts. She underwent a hysterectomy, and the uterus was preserved with its vascular supply. It was an orthotopic transplant with the donor uterus connected to the recipient's vaginal vault. Additional fixation was achieved by shortening the uterosacral ligament. The uterine arteries and veins were extended using reversed segments of the great saphenous vein, then connected to the external iliac arteries and veins, respectively. Immunosuppression was achieved with oral cyclosporine, azathioprine, and prednisolone. An episode of acute rejection was encountered. It was treated and controlled day with antithymocytic globulin. Following the transplant, an endometrial thickness of 18 mm was obtained with combined estrogen and progesterone therapy. The patient had two episodes of withdrawal bleeding upon cessation of the hormonal therapy. Ninety-nine days after the transplant, the patient developed acute vascular thrombosis for which hysterectomy was indicated.[2]

The second uterine transplant was done at Akdeniz University, Antalya, Turkey.[3] In this case, the uterus from a deceased donor was transplanted into a patient with Rokitansky syndrome.
The patient underwent in vitro fertilization (IVF) and embryo transfer 18 months after transplantation and had a miscarriage before 6 weeks.[3]
This was followed by 11 unsuccessful efforts to transplant and sustain a uterus in humans until the humungous success by Brännström et al. of Sahlgrenska University Hospital (Gothenburg, Sweden) in 2013.[4] A 35-year-old lady with Rokitansky syndrome category was chosen. A functional neovagina was created by mechanical self-dilatation.
IVF of the recipient and her partner was done prior to the transplant, and 11 embryos were cryopreserved after collection over three cycles.
The donor was a 61-year-old menopausal para 2 lady. She was treated for 3 months prior to transplant with a sequential combined oral contraceptive pills. Post the transplant, the recipient's first menstruation occurred 43 days, with cycles at regular intervals of between 26 and 36 days. One year after transplantation, the recipient underwent single frozen embryo transfer in a natural cycle, which resulted in pregnancy.
Triple immunosuppression (tacrolimus, azathioprine, and corticosteroids) continued throughout pregnancy.
Three episodes of mild rejection were observed but they were all reversed by corticosteroid treatment.
Fetal growth parameters and blood flows of the uterine arteries and umbilical cord were normal throughout pregnancy. She underwent a preterm delivery at 32 weeks due to preeclampsia and gave birth to a male baby with a normal birthweight for gestational age (1775 g) and with APGAR scores 9, 9, 10.[4]
INDICATIONS
Congenital
Mullerian agenesis.
Other mullerian anomalies not amenable to surgical correction or failed correction.
Acquired
Intrauterine adhesions, not amenable to or failed hysteroscopic surgical procedures.
Hysterectomy (peripartum) and for acquired indications.
SELECTION CRITERIA
Following are the revised montreal criteria for the ethical feasibility of uterine transplantation.[5]
The recipient
Has to be a woman of reproductive age with no medical contraindications to transplantation.
Should have documented congenital or acquired uterine factor infertility, which has not responded to the gold standard and conservative therapy.
Has a personal or legal contraindication to surrogacy and adoption measures and desires to have a child, or seeks uterine transplantation only as a measure to experience gestation, with an understanding of limitations provided by uterine transplantation in this respect.
Has not had her decision to undergo uterine transplantation deemed irrational by expert psychological assessment, and has no psychological comorbidity that interferes with diagnostic work-up or treatment.
Does not exhibit unsuitability for motherhood.
Is likely to take antirejection medication and follow-up with the treating team in a responsible manner.
Is responsible enough to consent, informed, and reliable enough to make a responsible decision and not under coercion.
The donor
Is a female of reproductive age with no medical contraindications to donation.
Has repeatedly attested to her conclusion of parity (no further desire to reproduce) or has signed an advanced directive for postmortem organ donation.
Has no history of uterine damage or disease.
Is responsible enough to consent, informed enough to make a responsible decision and not under coercion.
The health care team
Is part of an institution that meets Moore's third criterion (that innovative surgical procedures should be carried out in an institution with high overall expertise in the relevant domains) as it pertains to institutional stability.
Should have provided adequate information to both parties about risks, potential sequelae, and chances of success and failure.
Should have no conflict of interest independently or with either party.
Has the duty to preserve anonymity if the donor or recipient do not explicitly waive this right.
Transplant surgeons have to transplant and maintain a healthy uterus.
Gynecologists with expertise in high-risk pregnancies to perform the IVF and embryo transfer and manage the obstetrical issues of the mother during the pregnancy and postpartum.
Neonatologists, psychologist, social worker, and a psychiatrist.
LIVE VERSUS DECEASED DONOR
The uterine transplant donors can either be live or deceased (brain dead, beating heart). Each has its own advantages and disadvantages as follows:[6]
Advantages of a live donor
Can be chosen after matching.
Detailed preoperative evaluation of the medical status and pelvic evaluation can be done.
Potential emotional relationship of a known live donor and recipient exists.
Disadvantage of live donors
Increased surgical complexity and risks for the donor.
The organ retrieval from the live donor is tedious and time-consuming.
The length of the vessels available for anastomoses is short.
Advantages of a deceased donor
Surgical risk is not imposed on the donor.
Technically, a much simpler procedure as larger vessels can be used for anastomoses.
Organ availability is less.
Brain death induces major systemic inflammatory changes which may negatively affect graft survival.
Fibroids, endometrial polyps, hyperplasia, and cervical dysplasia that need to be ruled out prior to the procedure are not possible in case of a deceased donor.
PREOPERATIVE EVALUATION AND COUNSELING
Just like any tissue transplant, the uterine transplant too is a major surgery both for the donor and the recipient. The procedure should encompass a thorough evaluation to select the donor-recipient, match them and then counsel them duely to address any emotional and psychological issues. In general, the live donor has to be in good health to minimize the surgical risk at hysterectomy.[6] Imaging is done to rule out uterine pathologies, vascular anomalies, or atherosclerosis of uterine vessels. Blood grouping and Rh typing are done and matched between the donor and recipient.[6] The recipient is informed that she would need an IVF procedure in an ART laboratory to fertilize her oocytes obtained after controlled ovarian stimulation and ovum pickup (OPU) followed by embryo transfer once the transplanted tissue has been successfully tolerated by the body. IVF after surgery is not recommended as it is technically more difficult due to abnormal uterine vascular pedicles and anastomosis sites that increase the risk of bleeding at OPU and also due to the associated increased risk of infections in immunosuppressed patients. Pregnancy termination would be possible by a cesarean section. Surgical removal of the transplanted uterus could later be recommended for medical reasons, owing to rejection, surgical complications at cesarean section, or side effects of immunosuppression.[6]
PROCEDURE
One hour before retrieval of the uterus from the donor, surgery to prepare the recipient for transplantation is initiated in an adjacent operating theater.[4] The external iliac vessels are dissected after opening the abdomen with a midline incision and prepared for anastomosis. The vaginal vault is dissected from the bladder and rectum. The sutures that have to be used for uterine fixation are placed bilaterally through the round ligaments, sacrouterine ligaments, and the paravaginal connective tissues.
Uterus transplantation surgery involves isolation of the uterus from the donor with bilateral venous and arterial vascular pedicles. The dissection extends to the distal parts of the internal iliac veins and arteries. After surgical retrieval, the uterus is flushed cold histidine-tryptophan-ketoglutarate solution bilaterally through the arterial ends. Figures 1-3 diagrammatically represent the sites of vascular anastomosis between the donor and recipient.
The uterus is then brought into the pelvis. End-to-side vascular anastomosis, to connect the uterine veins to the external iliac veins, is done with 8-0 polypropylene sutures. Bilaterally, the anterior divisions of the internal iliac arteries are connected to the external iliac arteries using 7-0 polypropylene sutures. Blood flow to the uterus is ascertained by noting the color of the uterine tissue. The uterine graft is fixed to the ligaments, and the bladder peritoneum of the graft is attached to the recipient's bladder for extra structural support.[4] The most important aspect of any transplant is the postoperative immunosuppression. Related to the uterine transplant, the following immunesuppressive therapy is followed.[4]
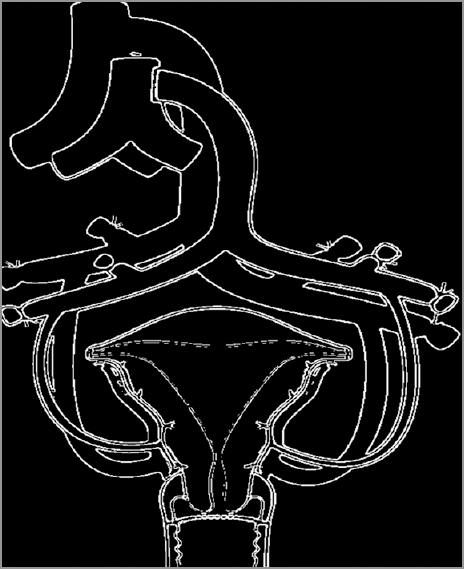
- Diagrammatic representation of the anastomotic site
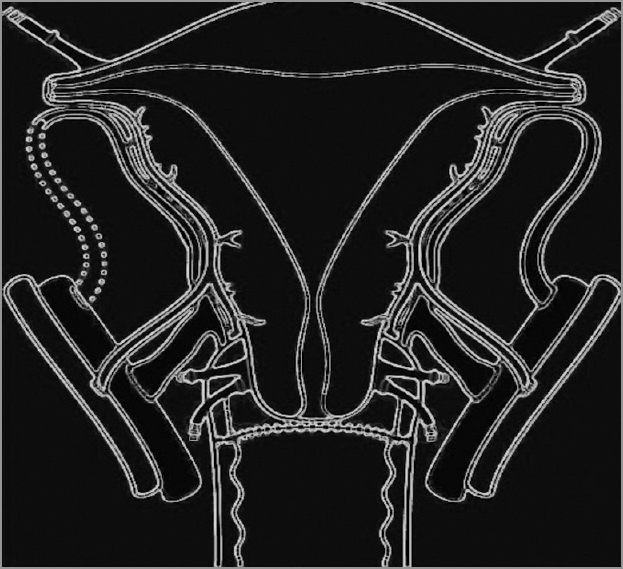
- End to side vascular anastomosis between the uterine veins and external iliac veins. Note the anastomosis between anterior division of internal iliac artery with the external iliac artery
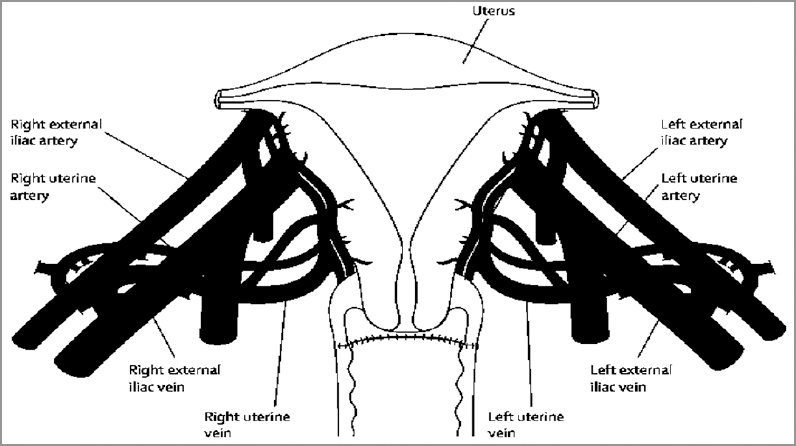
- Figure showing the anastomotic sites between the vessels of the donor and recipient
Immunosuppression
Antithymocyte globulin: 2·5 mg/kg just before surgery and 12 h later.
Methylprednisolone: 500 mg just before uterine perfusion.
-
Maintenance immunosuppression:
Oral tacrolimus 5-10 ng/mL.
Oral mycophenolate mofetil 40-60 mg/h/L during the first 10 months postsurgery.
Azathioprine 2 mg/kg/day used instead of mycophenolate mofetil after 10 months.
Prednisolone 5 mg daily added from month 6 posttransplantation because of repeated rejection episodes.
Follow-up
The patient is advised to follow-up twice weekly during the first postoperative month and then every 2 weeks in months 2-6. Antirejection monitoring is done as under:
Biopsy from transplanted vagina every 2 weeks for 3 months.
Endometrial biopsy every 3 months starting 3 months after transplant.
Urine and throat culture every month.
Sexual activity can be resumed after 3 months.
Once pregnancy is established, the patient is followed up every 2-3 weeks by specialists in high-risk obstetrics and transplantation.
RISKS ASSOCIATED WITH UTERINE TRANSPLANT
Surgical risks.
Risk of immunosuppressants to the mother and baby.
Rejection of graft.
Surgical risks
The surgery involves a long intraoperative time: More than 10 h for the donor and almost 5 h for the recipient which is associated with increased risks of anesthesia and prolonged immobilisation. The surgery is extensive and intricate involving many small vascular anastomosis in a particularly difficult and inaccessible part of the body. Transplant surgeries are associated with increased rejection risks, and long-term immunosuppression is needed. In case, biopsy confirms rejection of the graft, hysterectomy is indicated.
Obstetric risks
Following are the associated obstetric risks associated with uterine transplant:
Spontaneous abortion.
Premature births.
Intrauterine growth restriction.
Preeclampsia.
Delivery by elective lower segment caesarean section.
Azathioprine and tacrolimus in high doses are associated with congenital malformations in the baby.
ETHICAL CONSIDERATIONS
Despite the latest advances and progress made in this field, there are certain unanswered questions involving technical, legal, and social aspects of uterine transplantation.
Technical
Does it make sense to try to control the immune system's response with potent drugs that can cause life-threatening infections and cancers just for a few years and to hope for the recovery of that system through withdrawal of the medications and (presumably) the uterus?
Will the immune system recover back to baseline? Are experiences with failed kidney transplantation an appropriate model?
Are there long-term consequences for the patient?
Social
Are there really enough resources available to support widespread use of a therapy that is not required and may indeed be harmful?
If not, and it will only be available to wealthy individuals, how will they find a uterus?
Legal
Is it reasonable to intentionally expose a helpless fetus to development while receiving immunosuppressants? To the unknown impact of growth within a transplanted uterus? Who should consent for that fetus?
Average age at the time of the study - 31.9 years.
Average age at which surgeries were done - 28.5 years.
Only 13% had hysterectomies between 36 and 40 years.
Sixty percent had hysterectomies before the age of 30 years.
For those under 30, the average age at which hysterectomy was done - 24.6 years.
Of these 62% had bilateral salpingoophrectomy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Might uterus transplantation be an option for uterine factor infertility? J Turk Ger Gynecol Assoc. 2015;16:45-8.
- [Google Scholar]
- Ethical considerations in the era of the uterine transplant: An update of the montreal criteria for the ethical feasibility of uterine transplantation. Fertil Steril. 2013;100:924-6.
- [Google Scholar]
- Uterus transplantation: Animal research and human possibilities. Fertil Steril. 2012;97:1269-76.
- [Google Scholar]
- Medical Ethics: A case study of hysterectomy in Andhra Pradesh. Kics Forum.net.







