Translate this page into:
An outline on the aetiopathological association of genetic polymorphisms in women with female genital tuberculosis
Address for correspondence: Dr. Venkanna Bhanothu, Room No. F70, Department of Biotechnology & Bioinformatics, SLS, University of Hyderabad, Hyderabad-500046, Telangana, India. E-mail: banothu.venkanna@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The aetiopathological association of genetic polymorphisms in women with female genital tuberculosis (FGTB) is not understood completely. This review summarises the role of gene polymorphisms in Mycobacterium tuberculosis infection leading to infertility and switching on of the toll-like receptor 2 (TLR2) as well as the interferon-gamma (IFN-γ) signalling mechanisms and attempts to give information on amplification refractory mutation system (ARMS)–multi-gene (MG)/multi-primer (MP) polymerase chain reaction (PCR). The study was conducted in the Department of Zoology, Osmania University, Hyderabad, India. Desired articles for systematic reviews and meta-analysis strategies were used for the critically review. Keywords and internet searches were conducted in all electronic databases from the beginning of September 03, 2006 to July 07, 2017. Full-text, English language reviews and research articles based on FGTB, gene polymorphism and infertility were included. This review provides a comprehensive overview on the role of genetic polymorphism and mycobacterium infection in causing infertility, related symptoms and highlights the role of ARMS–MG/MP PCR for the detection of gene polymorphisms among infertile patients with FGTB. A total of 163 studies were recognised; only a minimum number of reviews (n = 4/163, 2.45%) scored well. A review on the association of genetic polymorphism in a well-characterised set of infertile patients with FGTB and healthy control women without tuberculosis was chosen as main outcome. This study noted that more research is needed to correlate mutations in TLR2 and IFN-γ along with the functional consequences of other factors and recommends considering the ARMS–MG/MP PCR for a rapid analysis of any known mutation in genomic deoxyribonucleic acid.
Keywords
Amplification refractory mutation system–multi-gene/multi-primer polymerase chain reaction
female genital tuberculosis
gene polymorphism
toll-like receptor 2 and interferon-gamma-mediated positive feedback loop mechanism
INTRODUCTION
Female genital tuberculosis (FGTB) is a persistent infection usually having a low-grade symptomatology with very few exact complaints. It is the foremost reason of childlessness and is a multifaceted condition with a myriad of causes, diagnosis and treatment. The rate of female infertility is rising and varies from 10 to 20%.[1] The worldwide incidence of infertility in genital tuberculosis (GTB) varies from 10 to 87%;[2,3] this condition is extremely prevalent in India,[4] with an incidence of 58%[5] and the majority of the patients are in the reproductive age (15–45 years) group.[6] The disease is predisposed for 5% of all female pelvic infections and arises in 15–20% cases of extra-pulmonary tuberculosis (EPTB).[7] In 80–90% cases, it involves women with menstrual abnormalities accounting for about 27% of the manifestations of FGTB.[8]
The pathogenicity and machinery of host reaction to FGTB is very divisive and still uncertain. The primary assault occurs with a few bacilli, which gradually inhabit and proliferate locally without causing symptoms. The primary infectivity of the genitalia may rarely arise from direct inoculation during sexual intercourse[9] with an infected cohort suffering from the tuberculous lesions of the genitalia.[10] The tuberculosis (TB) injuries are extremely dynamic and shaped by both, immune response elements and the pathogens. This co-evolutionary outlook emphasises the mutual shaping of the tissue microenvironment, which allows the breeding and transmission of Mycobacterium tuberculosis (M. tuberculosis) yet restricts tissue injure to safeguard the survival of the host. Once the genital tract is colonised, granulomata-containing viable tubercle bacilli form within various pelvic organs. After the development of tubercular hypersensitivity, these generally become clinically silent and intervals of 1–10 years or even longer may pass before infection at this site is reactivated or becomes clinically evident, if symptoms occur at all.[11] Once an active disease is established, then irreversible tubal and endometrial damage can occur and leads to the spread of granuloma to other organs. These are described as having compact, organised aggregates of immune cells consisting of blood-derived infected and uninfected macrophages, foamy macrophages, epithelioid cells (uniquely differentiated macrophages), and multinucleated giant cells (Langerhans cells) surrounded by a ring of lymphocytes. It generally remains silent, and physical signs are usually not present; hence, the disease remains mostly undiagnosed, or else, specific investigations are not commenced to rule out the problem. It is known that tuberculous foci may exist in the body and remain undetected for a long period.
The fallopian tubes are typically considered to be sterile; however, pathogenic microorganisms can come up from the lower tract (the cervix infection) and cause infection and inflammation in the upper female reproductive tract (FRT).[12] The stretch of the pathogen to the fallopian tubes, endometrial mucosa and ovaries leads to a variety of clinical conditions[11] such as infertility, irregular menstruation and pelvic pain. Clinical presentations such as oophoritis (inflammation of the ovaries) are also commonly seen in combination with salpingitis (inflammation of the fallopian tubes). During the pre-clinical stage, an insidious, low-grade inflammation is likely to modify the function of the fallopian tubes, uterus and endometrium, which leads to subtle damage and functional loss of the uterus and the tubes. The infection as it reaches the endometrium may persist in the basal layer, not shed during menstruation and/or infects the tubes following menstruation. In the cervix, the tuberculous lesion can be ulcerative or proliferative or may present as a mass mimicking malignancy.[13] The lesions in the cervix and vagina are usually present as isolated, chronic, ulcerative lesions.[14] In ulcerative form, the ulcers have serpiginous outline, clean-cut edges and a yellow base. Early ulcers are often seen near the opening of the central cervical canal into the vagina (i.e. external os). The signs, symptoms and the altered immune responses of patients with FGTB imitate those of immunosuppressed persons; therefore, diagnostic attentiveness may prevent unnecessary treatment and morbidity.[15]
Immune defence system of the reproductive tract
Although bacterial infections are predisposed by environmental factors, a range of strong host genetic factor heaviness is also likely during the interaction between humans and potential bacterial strains through the immune system and inflammatory signalling pathways, consequential in host immune responses and inflammation.[16] The host immune response against pathogen is orchestrated by the innate and cellular arms of the immune system along with antibody-mediated immunity. Furthermore, the innate immune system of the FRT is categorised in to mechanical, chemical and cellular components.[17] The anterior urethra and cervicovagina are the only anatomic areas of the genital tract system perpetually colonised with microbes and termed as the reproductive tract microbiome.[18] The mucus lining and epithelial cells along with protective microbiome act as the mechanical barriers. Besides being a physical barrier for microorganisms, the mucosal epithelial cells may actively contribute in the mucosal immune defence by secreting cytokines in response to pathogens.[19] The chemical barriers are natural anti-microbial peptides and pattern recognition receptors (PRRs)[20] that offer the first line of defence against the invading classes of pathogens and incorporate immune response quickly after dealing with infectious agents. As a consequence, the activation of adaptive immune system takes place by improving the surface defences, cytokine elaboration, cellular complement activation and phagocytic responses[21] as a part of host defence.[21,22]
The innate immune system has the ability to recognise the structurally conserved distinct products of microbial metabolism present in a wide variety of microorganisms and mediates the immune responses. These conserved products, termed pathogen-associated molecular patterns (PAMPs),[23] are recognised by germline-encoded membrane-bound receptors expressed mainly on the host immune cells termed PRRs.[21,24] A cascade of events occur following PAMPs recognition by PRRs, which activates host defence mechanism to prevent or fight off infections and subsequently initiate signalling and enhance adaptive immune response.[25]
Many functions of innate immunity are transduced through PRRs, which recognise invading microbes and activate signalling pathways that initiate immune and inflammatory responses to destroy the invaders or pathogens. Recent studies have consigned a critical role to one particular group of PRRs including the evolutionarily conserved mammalian toll-like receptor (TLR), a family of extremely similar proteins.[26] TLRs are a family of PRRs consisting of 13-member eukaryotic receptor forms, as of an ancient gene group, which is found in invertebrates and vertebrates.[21,27] In humans, ten functional TLRs (TLRs 1–10) have been recognised.[28] TLRs 1–6 have constantly been reported in human and mouse reproductive tract epithelial cell lines (endocervical, ectocervical, vaginal and uterine cell lines) and endometrial epithelial cells.[29,30,31] The expressions of different TLRs in the FRT are summarised in Table 1.[32,33] The expressions of TLR1-10 genes are also reported in the human endometrium and endometrial tissue (excluding menstruation samples), and the mean relative expression of TLR2–6, 9 and 10 genes was reported to be significantly higher during the secretary phase when evaluated in comparison with other phases of the menstrual cycle.[31,34]
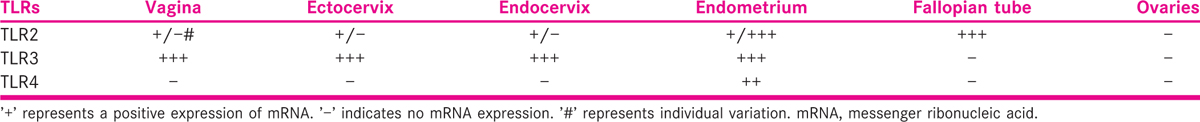
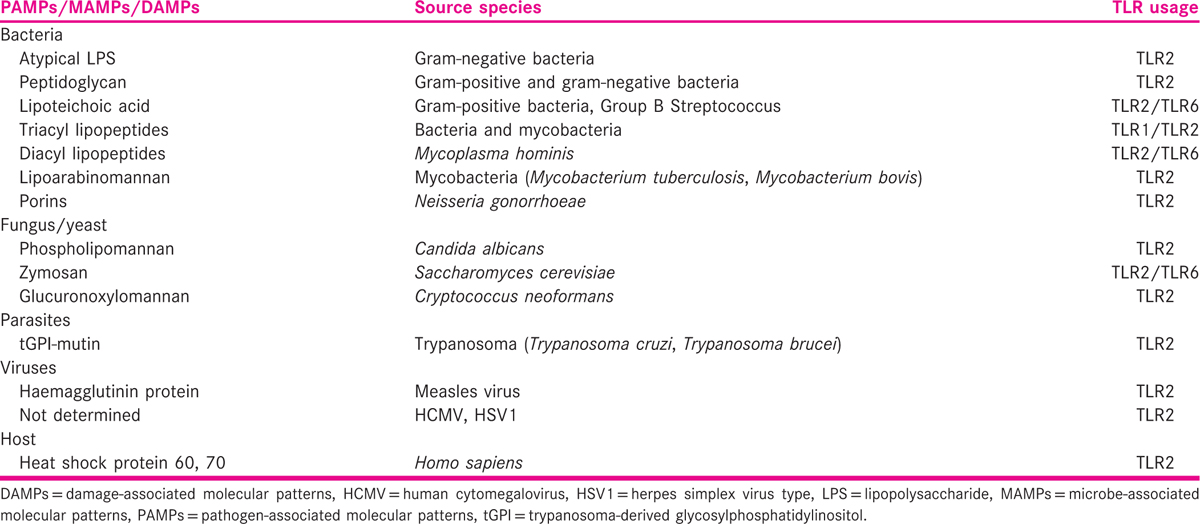
The pattern recognition of M. tuberculosis is a complex process initiated by bacterial contact with macrophages, in which, the multitude of receptor proteins identifies specific components of the microorganism.[35] The recognition of mycobacteria by specific receptors leads to the up-regulation of diverse intracellular signalling pathways, to integrate and induce an efficient signalling in the activation of the innate host defence mechanism and the subsequent overture of adaptive immune response. Among all the functional TLRs described in humans, the cytoplasmic domain of TLR2 is exceptional in its requirement, because it forms functional heterodimeric complexes with either TLR1 or TLR6 for the instigation of cellular signalling, cellular response, immune modulation and the immune regulation of reproductive functions. TLR2 does not form a homodimer by itself; thus, TLR2 is considered to be the most vital receptor in identifying a vast array of ligands, including a broad range of microbial products from gram-positive bacteria (peptidoglycan),[36] fungi (zymosan),[37] bacterial lipopeptide[38] including synthetic lipoproteins S-[2,3-Bis(palmitoyloxy)propyl]-N-palmitoylcysteinyl-seryl-lysyl-lysyl-lysyl-lysine (Pam3Cys-Ser-(Lys)4)[39] and eventually leading to a cascade of signalling responsible for cytokine induction [Table 2]. TLR2/TLR1 and TLR2/TLR6 heterodimeric forms have been implicated mostly in the detection of mycobacterial cell wall glycolipids, and lipopeptides/lipoproteins such as 38-kDa protein, LpqH (19-kDa lipoprotein, Rv3763),[40] lipoprotein signal peptidase (LspA), lipoprotein-LprA (Rv1270),[41] lipoprotein-LprG (Rv1411c),[42] triacylated lipoproteins or diacylated lipoproteins[43] consequently trigger cascade signals.[44] Other than the lipoproteins, TLR2 also interacts with lipopolysaccharides, lipoteichoic acid, lipoarabinomannan, lipomannan,[45] peptidoglycan and phosphatidylinositol mannoside[46] along with endogenous ligands (heat shock proteins) released by damaged or necrotic host cells[47] to initiate the cellular responses.[46] However, a recent study showed that only lipoproteins/lipopeptides were ‘true’ TLR2 ligands and accepted as ligands at physiological concentrations.[48] It was also measured that the differential immune responses were mediated by totally distinct chemical structures of mycobacterial components and the dimerisation, heterodimeric rearrangements and conformational transforms of TLR2 receptor in response to ligand interaction.[49]
Toll-like receptor 2 and interferon-gamma genes polymorphism
TLR2, a protein that in humans is encoded by the TLR2 gene,[50] is also designated as cluster of differentiation factor (CD) 282. TLR2 is a very attractive receptor that plays a crucial role in pathogen recognition, essential for the initiation and coordination of the innate immune responses to distinguish the pathogens[49,51] and enhance the adaptive immunity cascades of effector responses against pathogens.[52] Thus, TLR2 is known as the key sensor of mycobacterial infections. After infection with M. tuberculosis, primary host immune response cells, such as the dendritic cells (DC) and macrophages, engulf mycobacteria by phagocytosis and present pathogen-derived peptide antigens to naive T lymphocytes (mature in the thymus) (T-cells). Host innate immune cells sense the existence of mycobacterial-derived components through the activation of TLR2. Although the interaction of M. tuberculosis with TLRs leads to phagocyte activation, the communication itself does not lead to the immediate ingestion of the mycobacteria. Subsequent to the interaction of specific mycobacterial structures with TLRs, the outcome of signalling through TLR2 can be explained in part by the selective usage of five types of adaptor molecules namely myeloid differentiation primary response gene 88 (MyD88), toll/interleukin receptor-domain-containing adaptor protein (TIRAP)/MyD88-adaptor-like (MAL), TIR-domain-containing adaptor-inducing interferon (IFN)-β (TRIF)/TIR-domain-containing molecule 1 (TICAM1), TRIF-related adaptor molecule (TRAM) and sterile alpha- and armadillo motif-containing protein (SARM). Macrophages and DC sense the presence of mycobacteria through the activation of TLR2.
This results in the production of cellular mediators such as interleukin (IL)-1, IL-6 and IL-12 that in turn activate CD4+ and CD8+ T-cells. Activated T-cells produce IFN-γ and other mediators that result in the positive feedback loop (IL-12 → T-cell → IFN-γ) mechanism involved in the activation of macrophages to kill intracellular pathogens in later events, as are shown in Figure 1.[53,54,55,56,57] Strong evidence is available for the selection of genetic variants in modulating infectivity and differential clinical presentation for genes encoding several types of proteins[16] including macrophage receptors, soluble C-type lectins, TLRs,[58] major histocompatibility complex/human leucocyte antigen,[59] cytokines and chemokines.[60] Among these, TLR2 and IFN-γ genes are most important, because TLR2 signalling pathway contributes to IFN-γ production.[61,62,63] TLR2 gene in particular plays an important role in pathogen recognition, initiating the signalling cascades and arbitrates the interactions between the immune and reproductive systems[64] in response to pathogen. Holland and Casanova described the role of IL-12 and IFN-γ in the stimulation of macrophages and T-cells in response to mycobacterial infections.[65] Upon infection, primary host response cells such as macrophages release a range of cytokines including IL-12, which stimulates T-cells or natural killer (NK) cells to produce IFN-γ. IFN-γ activates macrophages to destroy intracellular pathogens and enhance the differentiation of IFN-γ-producing T helper cells. The risk of developing TB has been shown to be associated with arginine 753 glutamine (R753Q) polymorphisms in the TLR2 gene,[66,67] and more specifically with shorter guanine-thymine (GT) repeat polymorphism in intron II of the TLR2 gene. However, earlier studies have reported the lower expression of TLR2 protein[68] on their coexistence.
![The toll-like receptor 2 and interferon-gamma-mediated positive feedback loop mechanism in response to M. tuberculosis: expression of immune response genes in the macrophages/dendritic cells leads to activation of natural killer cells/T cells for the production of IFN-γ, other mediators and elimination of bacilli.[37,53,54,57]](/content/172/2016/3/2/img/FSAR-3-66-g003.png)
- The toll-like receptor 2 and interferon-gamma-mediated positive feedback loop mechanism in response to M. tuberculosis: expression of immune response genes in the macrophages/dendritic cells leads to activation of natural killer cells/T cells for the production of IFN-γ, other mediators and elimination of bacilli.[37,53,54,57]
TLR2 plays an important role in protecting the host from various pathogens including M. tuberculosis.[69] However, single nucleotide polymorphisms (SNPs) in the human TLR2 gene have been linked with a decreased response to various bacterial lipoproteins and septic shock following a gram-positive bacterial infection. TLR2 polymorphisms associated with TB signify the regulatory variants rather than non-synonymous SNPs that alter the amino acid sequence.[70] The patients heterozygous for arginine 753 glutamine (R753Q) had reduced production levels of IFN-γ signifying that altered TLR2 responsiveness might contribute to the course of infections[66,69,71,72] including TB.[66,67,70,73] In human, SNPs located in the first intron of the IFN-γ gene (at position +874) have shown variable associations with disease susceptibility and severity.[74] There are two well-known SNPs in the IFN-γ gene non-coding region (intron 1): +874A/T polymorphism and CA microsatellite. SNPs are single-allele mutations [deoxyribonucleic acid (DNA) sequence variations that occur when a single nucleotide [adenine (A), T, cytosine (C) or G] in the genome sequence is altered] in the genomic sequence of an organism, which are responsible for about 90% of all human DNA variation and play significant role in human evolution, drug sensitivity and disease susceptibility.[75] Pravica et al. revealed a novel SNP,[76] T to A, located at the +874 position from translation start site in the first intron of IFN-γ gene, which coincides with a putative nuclear transcription factor-kappa light-chain enhancer of activated B-cells (NF-κB)-binding site that could play a essential role in the induction of constitutively high IFN-γ production. The differences in the magnitudes of the responses that were seen may reveal the environment in which the cohorts live, or they may reflect the nature of the patients’ infections.[77] The disruption of the IFN-γ gene in mice infected with M. tuberculosis has resulted in the exacerbation of disease, progressive and extensive tissue destruction and necrosis with numerous bacteria.[78] Therefore, alteration in IFN-γ production may influence the susceptibility to FGTB, and this alteration could be due to gene polymorphism. The homozygous T/T, A/A and heterozygous A/T alleles are associated with increase or decrease in the production of IFN-γ, which can affect the outcome of the disease severity. Recently, the SNPs of TLR2 G to A substitution at position 2258 (2258G/A) [arginine to glutamine substitution at residue 753 (Arg753Gln)] and IFN-γ [T to A substitution at the +874 position located from the translation start site (+874T/A)] gene that leads to a decreased response of macrophages to microbial peptides associated with receptor hypo-responsiveness and attenuated immune response with the host have been reported.[66,74,76,78,79] TLR2 gene polymorphism results in an arginine (CGG) to a glutamine (CAG) substitution, and the resulting genotypes, therefore, are arginine/arginine (GG), glutamine/glutamine (AA) and arginine/glutamine (AG). The mechanisms through which these TLR2 polymorphisms affect host defence remain unclear. This apparent variability is probably accounted for by the involuntary selection of differential clinical presentations as a result of long-term host–pathogen interactions in certain regions or populations. However, the identification of factors involved in the aetiology and physiology of multifaceted disorders and conditions such as infertility is needed to understand potential regulatory mechanisms involved in disease pathogenesis. The determination of functional SNPs and biochemical phenotypes (e.g. the differential expression and function of innate immune molecules such as receptors, secondary mediators and cytokines by the host) in the immunopathogenesis of FGTB is necessary in assessing the contributions and functional consequences of specific genetic variations (polymorphisms) in the human genome with reverence to host susceptibility or resistance to TB.
Amplification refractory mutation system–multi-gene/multi-primer polymerase chain reaction
Mutation screening is one of the miscellaneous technologies useful to identify mutations or polymorphisms in candidate genes or genomic regions. Because the human genome sequence is available, polymerase chain reaction (PCR)-directed sequencing is adapted as one of the best methods for mutational analysis. Allele-specific PCR or amplification refractory mutation system (ARMS)–PCR is an ordinary technique used for the detection of point mutations or small deletions in several studies.[80] Unfortunately, assay design can be laborious if multiple genes or large regions need to be studied by simple PCR. Bhanothu et al. improved the ARMS–PCR to permit the rapid analysis of any known mutation in genomic DNA and established a system, ARMS–MG/MP PCR, which allows genotyping exclusively by the examination of reaction mixtures after agarose gel electrophoresis.[81] The principles and applications of MG/MP PCR and/or ARMS–multi-gene (MG)/multi-primer (MP) PCR in the clinical diagnosis of FGTB and mutational analysis are described elsewhere.[80,81] The ARMS–MG/MP PCR consists of multiple primer sets within a single PCR mixture to produce products of varying sizes that are specific to diverse DNA sequences in a single tube reaction. MG/MP PCR method uses a single template along with several pairs of forward and reverse primers to amplify specific regions within a template. Amplification is also attainable with multiple templates or target sequences and several primer sets in the same reaction tube.
Potential problems in a simple PCR include false negatives due to reaction breakdown or false positives due to contamination. False negatives are often exposed in MG/MP PCR assays because each amplicon provides an internal control for the other amplified fragments. This problem can be overcome by designing or picking dedicated primers. The cost of reagents and preparation time can be less in MG PCR compared to other systems in which several tubes of uniplex PCRs are used. MG/MP PCR reactions decrease the cost by minimising the use of polymerase and templates. The quality of the template may be determined more effectively in a MG PCR than in a simple PCR reaction. This study aims to review the role of TLR2 and IFN-γ gene polymorphisms in M. tuberculosis infection leading to infertility and the role of TLR2-IFN-γ signalling immune defence mechanism. The review also brings out the importance of amplification refractory mutation system (ARMS)–MG/MP PCR and its role in the rapid detection of SNPs among latently infected individuals who are at risk of developing active TB and infertility.
MATERIALS AND METHODS
Collection of literature and analysis
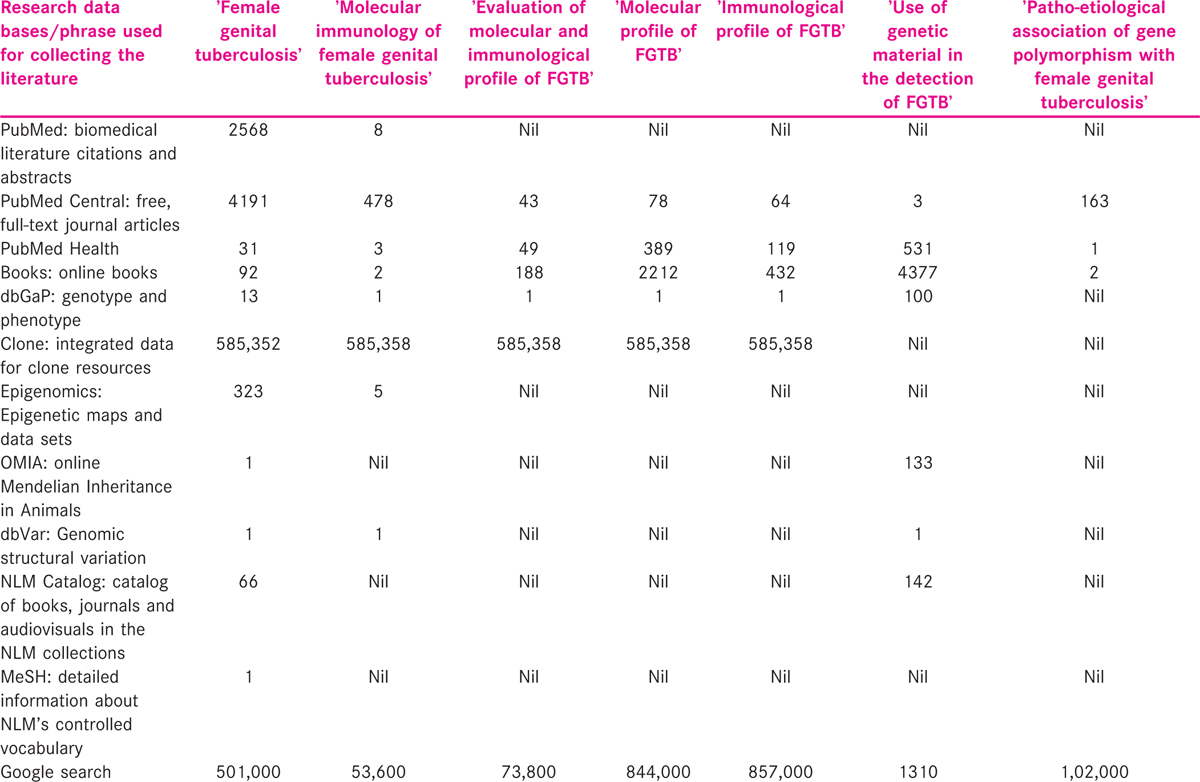
A systematic literature review was performed as part of a study aiming to know the gaps and available facts on the aetiological and pathological association of genetic polymorphisms in women with FGTB. The compilation of literature and revise of literature were conceded at the Department of Zoology, Osmania University, Hyderabad. This centre is connected with high-speed internet facilities permitted to access all international and national journals, e-journals and books. The exploration of facts was performed from September 03, 2006 to July 07, 2017 from all data sources including MEDLINE and the Cochrane Library (Wiley) from the date of inception, with no language limits. Several internet-associated search engines such as Pubmed-NCBI, Google and sources such as libraries were used for gathering associated data. Research articles were retrieved through search engines using specific terms such as ‘Female genital tuberculosis’, ‘Molecular and immunological factors of female genital tuberculosis’, ‘Use of genetic material in the detection of FGTB’ and ‘Etiological and pathological association of gene polymorphism with female genital tuberculosis’. Retrieved research articles, review articles, chapters from books, letters, editorials, commentaries, dissertation, books, erratum, notes, introductory, meeting abstracts, case reports, guidelines, conference proceedings, seminar presentations and abstracts were systematically studied. The related information from the articles were snowballed, corrected and presented. Quality evaluation was not an insertion criterion, because the aim was not to verify the gene polymorphism in the given population but to know the gaps, facts and its relationship with infertility and mycobacterium infection. Sample population included in the study was from reproductive age stratum. Excluded manuscripts were those describing studies without the information of gene polymorphism, FGTB and infertility. Enormous information got out of exploration were collectively filtered and selectively stored, and the rest of the unwanted literature was excluded.
RESULTS
Data from published manuscripts relating to randomised controlled trials, pilot and expediency studies of genetic association with tuberculosis susceptibility were gathered. Data were extracted from eligible manuscripts pertaining to the pathological and aetiological association of genetic polymorphisms in women with FGTB. Using Google search engine (https://www.google.co.in/#hl=enandoutput=searchandsclient) for about 1,02,000 citations, the title ‘Patho-etiological association of gene polymorphism with female genital tuberculosis’ was noted. Using PubMed-NCBI search engine (http://www.ncbi.nlm.nih.gov/pubmed), about 163 articles were identified, out of which, four were connected to the topic of investigation.
About 1310 citations were noted by title ‘Use of genetic material in the detection of FGTB’. Using PubMed-NCBI search engine (http://www.ncbi.nlm.nih.gov/pubmed), about four articles were documented, out of which, three were associated to the topic of investigation. The details of search engine outcomes are specified in Table 3. The outcome of the survey showed very little information about ‘Patho-etiological association of gene polymorphism with female genital tuberculosis’ and ‘Use of genetic material in the detection of FGTB’. However, unrelated information was also seen during the search and was discarded. Therefore, this indicates that there is a need to provide quality information on the mutational association of FGTB in an approach to enhance the research in this area. This review article attempted to summarise the use of technological developments in the detection of gene polymorphism and FGTB disease among infertile patients.
DISCUSSION
FGTB usually occurs in women <40 years of age[82] and may simulate ovulation dysfunction that often presents with absent, excessive or non-cyclical menstruation.[83] Even foetal growth retardation was noted in several studies due to TB.[84,85] It is a major cause of sub-fertility and infertility and is rarely diagnosed.[5,86] The aetiology of infertility is an important criterion for the identification and categorisation of GTB in infertile women.[87,88] Similarly, Bhanothu et al. reported a strong relationship between FGTB and infertility among women with diverse clinical presentations.[89] The significance of FGTB among all forms of TB has not yet been determined in developing countries. Such a situation is due to the complexity in clinical diagnosis, which is possible by laparoscopic investigations or endometrial biopsy or the culturing of M. tuberculosis or hispathologic analysis or MG/MP PCR,[89,90,91] but are not always available. Such as circumstance is also expected due to the lack of reliable data and strategy for early diagnosis. All organs are exaggerated by EPTB; however, the recurrence of the diverse clinical spots varies with region to region.[89,92] The reason why some organs are affected aetiologically and pathologically more than others in these scenarios is not well understood. However, as detected in the pre-antibiotic era, the EPTB often has a favourable outcome[89,93] when compared to PTB.[94] Therefore, the early detection of the disease and the identification of factors associated with FGTB susceptibility are of current importance.
Intricate interactions between host and pathogen have been described to affect the outcome of disease, including clinical severity, susceptibility and the latent forms of infections, and subsequently the development of infertility. Once the immunity to M. tuberculosis contains and overrules the growth of a small number of disseminated TB bacilli after primary infection, the infection may remain silent throughout the life until overwhelming re-infection precipitates the disease due to lowered immunity.[95] Patients in whom the infection converts into disease, cavitary lesions are developed and the bacteria increase in number in the caseous detritus. Patients with such a type of lesion are highly infectious,[96] and the classic symptoms of GTB such as persistent inflammation associated with bloody vaginal discharge, postmenopausal bleeding, pelvic pain, menorrhagia, oligomenorrhea, dysmenorrhoea, beaded tubes, tubal blocks, hydrosalpinges, tubo-ovarian mass and failure of implantation are noted. Menstrual disturbances are more likely when the female genital tract (FGT) is involved in tubercular process. The infections depend on the site of inflammation,[91] and the spread of pathogen to the reproductive organs leads to a variety of clinical conditions[11] including infertility. In fact, fertility is also profoundly affected, not only by systemic infections that directly or indirectly target the reproductive organs, but also by various illnesses including intrauterine growth restriction, pre-eclampsia, preterm birth, recurrent pregnancy loss, endometritis, endometriosis[97,98] polycystic ovaries syndrome, ovarian cancer, chronic inflammatory conditions initiated due to genetic polymorphisms or genetic alterations and abnormalities in various genes and chromosomes.[66,69,73,81,99,100] A number of studies on susceptible genes and genome-wide linkage scans[101] have identified the association of several functional SNPs to TB susceptibility.[16] Despite the significant role that TLR2 and IFN-γ plays in immune defence and human reproduction, little is known about the polymorphism of these genes[64,73] in infertile women with FGTB.[81] A mutation in TLR2 specifically inhibited M. tuberculosis-induced cytokine production, and this inhibition was incomplete and thereby suggested that beside TLR2 polymorphism other genes may be involved.[37] Yet, there is no direct evidence that this polymorphism causes a decline in immune response specifically to M. tuberculosis. Polymorphism in genes encoding cytokines such as IFN-γ, tumour necrosis factor-α (TNF-α) along with other immune mediators has been associated with increased susceptibility to TB in different populations. This may also play a role in the pathophysiology of the disease.[67,74] Gene mutations in the TLR2 pathway are also reported in persons with M. tuberculosis[67,102] infections. Darville et al. reported genital tract infection by C. trachomatis in transgenic mice lacking TLR2.[103] In the TLR2 knockout mice, significantly lower levels of the inflammatory cytokines, TNF-α and macrophage inflammatory protein-2 have been reported in the genital tract secretions. Despite the variation in cytokine production, the course of infection, however, remained the same in all animals with sufficient effectors cells recruited to the genital tract to clear the infection. The reduced pathology in TLR2 knockout mice is of particular interest; however, the animals showed decreased inflammatory cell infiltration, dilatation and hydrosalpinx within the fallopian tubes. The TLR2 2258A is reported to have a significant decrease in NF-κB response against bacterial peptides in human embryonic kidney (HEK)-293 T-cells transfected with wild type or TLR2 2258G/A constructs.[66,104] The progression of the disease in chronic oviductal pathology mediated by TLR2 gene polymorphism is also reported.
Despite these, Bhanothu et al. used MG/MP PCR to determine whether certain organs had an affinity for M. tuberculosis.[89,93] Bhanothu et al. also explained the relevance of TLR2 and IFN-γ genes in the context of varied clinical presentations with the site-specific pathology of TB infection[81] and partly described the tissue-specific expression of gene polymorphisms and genetic abnormality leading to atypical gene regulation[105,106] in infertile women with FGTB. The genotype distribution of TLR2 gene is not differing significantly between the patients with FGTB and healthy controls. There is no evidence of an association between the TLR2 gene polymorphisms and its role in inducing infertility among patients infected with M. tuberculosis and control patients without TB.[81] Bhanothu et al. suggested that TLR2 gene polymorphism may not be the influencing factor in the disease susceptibility, but defects in the factors other than TLR2 are suspected in infertility and in inducing latent TB among such infertile patients.[81] Bhanothu et al. could probably suggest a limited role of the TLR2 gene and its polymorphisms in disease susceptibility.[81] However, TLR2 (R753Q) polymorphism has been described in association with susceptibility to several infectious diseases. Many functional and genetic studies confirmed that SNPs in TLRs have the ability to both modify receptor function and increase the susceptibility of the host to TB infection. So far, no functional domains have been identified in human TLR2, but the potential functions of the carboxy terminus include dimerisation to form TLR2 homodimers or heterodimers with downstream targets such as adaptor molecules namely MyD88, TIRAP/MAL, TRIF/TICAM1, TRAM and SARM. Infections in animal models suggest that the haematogenous spread of infection occurred before the onset of T-cell-mediated immunity.[107] This supports the hypothesis that the ability of the different strains of M. tuberculosis to produce different clinical phenotypes depends upon their interaction with the host innate and adaptive immune responses, but the relevance of these findings to human disease still remains uncertain.[108] However, it is possible that more common genetic variants such as promoter region polymorphisms that influence gene expression may be associated with certain diseases. The heterogeneity in the clinical expression of FGTB strongly suggests the role of genetic and environmental factors in immune pathogenesis along with aetiology. This also affects the differential expression of genes such as TLRs, cytokines, chemokines and lymphokines found in the host immune response and human reproductive system.[109] The manifestations of clinical presentations such as beaded tubes, tubal block with hydrosalphinx, tubercular salphingitis and omental adhesions along with menstrual complications are correlated due to genetic variations in the bacterial strain and the extraordinary virulent nature of mycobacterium including the genetic makeup and genetic mutation of the host.
Infertile women with M. tuberculosis infection have the standard levels of TLR2 expression, which may be sufficient to induce a local Th1-type host defence (i.e. IFN-γ production) against the infections. However, fluctuation in the production of IFN-γ and its role in pathogenesis have been described to be due to specific mutation in IFN-γ +874A/T gene.[76,110,111] IFN-γ+874(T/A) polymorphism lies within the transcription factor-binding site of the NF-κB and is responsible for the unusual binding pattern of specific alleles. The mechanism by which the IFN-γ +874T/A allele influences the susceptibility to FGTB may depend on its role in the regulation of IFN-γ production. The T allele of IFN-γ +874T/A presents a binding site for the NF-κB, which is able to regulate IFN-γ expression.[76] Heterozygous carriers have a transitional phenotype, more subtle variation in the IFN-γ response pathway and may underlie the susceptibility to TB in human populations.[112] Mice deficient in the IFN-γ gene expression are very susceptible to fatal TB.[110,113] The higher levels of IFN-γ production along with other cytokines suggest the role of cytokines in infertility, diagnosis and treatment.[114] Mutations that modulate the IFN-γ production have been coupled with different clinical presentations,[115,116] outcome of active TB and in the aetiology of female infertility due to mycobacterial infections.[117,118] The endogenous ligands identified in other systems have suggested the significant role of TLRs in the normal functioning of the ovary and endometrium through the menstrual cycle. The remarkable levels of IFN-γ may be due to genetic variations that lead to the susceptibility of TB infections and infertility. M. tuberculosis-induced IFN-γ seems to be the downstream effectors molecule of TLR2 activation results in the mitigation of TB induced infertility among women. Bhanothu et al. suggest that a deficient IFN-γ signalling in infertile women could lead to the loss of bacterial infection-mediated protection of the genital organs.[81] M. tuberculosis-induced IFN-γ production depends on TLR2 signalling and thus exerts regulatory effects on fertility in women with FGTB. TLR2-IFN-γ signalling pathway may also yield novel insights into the regulatory mechanisms of immune cells in infertile women associated with genital infections such as FGTB. Bhanothu et al. reported that the distribution of IFN-γ gene polymorphism is consistent with earlier information; however, the distribution of TLR2 gene polymorphism is different from the earlier findings.[73] Bhanothu et al. noted a significant correlation of the disease with IFN-γ gene polymorphism but not with TLR2 gene polymorphism.[81,111] Bhanothu et al. also had shown the role of TLR2 gene polymorphism in association with disease resistance in humans. Therefore, the genetic variants of TLR2 and IFN-γ can be utilised as biomarkers in the early detection of women with FGTB.
Disparity in the statistical distribution of polymorphism may be due to an extended duration of infertility, polluted environment and the nutritional status of individual. Limited information is available on gene polymorphism in association with infertility, and this may perhaps confine our understanding about disease and statistical disparity in the genotype. Validations of the findings among independent cohorts are needed to firmly establish the role of TLR2 gene polymorphism in conditions such as organ dysfunctions and infertility with varying clinical presentations. A final proof of their functional relevance requires further studies that could determine their realistic effects on immune response. This review concluded that the study by a larger cohort of infertile patients with FGTB is warranted to identity the risk factors and candidates or susceptible genes. This review also supports the perception that genetics, immunology, environment and different strains of infecting agents may play a vital role in the aetiology and pathogenesis of FGTB among infertile patients. The review provides conceptual advances useful in the understanding of the onset and progress of TB. Further, this review also suggests that the identification of the asymptomatic nature of the disease, the accessibility of reproductive clinics, the elucidation of genes associated with virulence and susceptible factors, the detection of interspecies and intraspecies differences among genome sequences and gene expression are importance attempts to understand the aetiology and pathogenesis of FGTB disease.
TLR2 receptor is extremely implicated in the field of adjuvant, pathogen and probiotic agents at the point of entry. IFN-γ is involved in carrying the TLR2 signalling cascades and immune modulation in the event of pathogen elimination. The determination of functional SNPs and biochemical phenotypes (e.g. the differential expression and functions of innate immune molecules such as receptors, secondary mediators and cytokines by the host) in the immunopathogenesis of FGTB is required. It is then possible to differentiate the early and late stages of the disease by identifying stage-specific biomarkers that could differentiate between each stage. It is, therefore, necessary to screen a variety of candidate genes, such as those for cytokines and the members of the TLR2-IFN-γ-mediated signalling pathway with positive IFN-γ/IL-12 feedback loop. This also supports the hypothesis that the functional polymorphism in the gene-encoding TLR2 function may not be a rate-limiting component for containment by the human innate immune system and natural gram-positive bacterial infections (due to extra- and/or intracellular pathogens) along with the role of other factors. Therefore, more research is needed to correlate mutations in TLR2 and IFN-γ with functional consequences.
An improved understanding of innate and adaptive immunity within the FRT may help to develop interceptive strategies against the pathogens and clinical outcomes. By understanding the physiology and molecular mechanisms that govern M. tuberculosis infection and host cell interactions with specific bacterial molecules at different stages of infections can be targeted for rational of FGTB diagnosis and strategies to control TB infections. Advanced nucleic acid-based methods such as MG/MP PCR can be utilised not only for bacteriological presence but also in the clinical findings of host in response to infectious agents. This technique on slight modification can be used in the detection of SNPs, mutations, gene deletions and in the comparative analysis of various biomarkers. However, sex disparity in TB notification rates have been stated frequently, with a higher incidence in males. This discrepancy is commonly explained by either under-diagnosis or under-reporting of TB in females. A study of the sex ratio among patients with TB in San Francisco,[119] on the basis of clustering statistics, recommended that the sex divergence may be due to a difference in transmission dynamics rather than under-diagnosis. The variation in transmissibility and virulence among M. tuberculosis strains has been related to the genetic background, diverse lineages with specific geographical regions and the ethnicity of the host population.[120,121] It may be due to an inconsistency in infection and/or the progression of disease.[122] A broad range of gender disparity was noticed among the patients with GTB infections, with 78.5% of the patients being females.[93] However, the rate was comparatively low (42.8%) in female patients with PTB <21 years old and increased significantly in patients more than 21 years old and reached 89% in patients between 31 and 40 years old.[123] The global rate of clustering in female patients was higher than that in male patients. A high rate of clustering is associated with recent infection and rapid progression to disease.[124] There may be numerous explanations for the higher rate of clustering in middle-aged women. It is likely that women are more disturbed by immunodeficiency, and women may progress more rapidly to diseases.[122,125] Because women’s health has an impact on both their families and the economy, the inclusion of sex difference in TB control programs could yield more beneficial outcomes.[126] It is more likely that socio-economic and cultural factors (poverty and the crowded state of life, individual’s age, environment, lifestyle, nutritional status, close pregnancies, prolonged breast-feeding and so forth) affect the rates of TB infection. Therefore, understanding the multifaceted role of one’s genotype, environment and age with changes in one’s epigenotype could further sort the unknown causes of the disease. The correlation of epidemiological examinations along with the aforementioned factors could provide a greater understanding of different gene regulatory pathways and their effect[127] on human diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We are grateful to National Post Doctoral Fellowship scheme, SERB-DST, Govt of India for providing research fellowship and grants in sciences for meritorious scientists.
REFERENCES
- Female genital tuberculosis and infertility. Int J Gynaecol Obstet. 2001;75:269-72.
- [Google Scholar]
- Infertility and pregnancy outcome in female genital tuberculosis. Int J Gynaecol Obstet. 2002;76:159-63.
- [Google Scholar]
- Genital tuberculosis in Indian infertility patients. Int J Gynaecol Obstet. 2007;97:135-8.
- [Google Scholar]
- Role of latent genital tuberculosis in repeated IVF failure in the Indian Clinical setting. Gynecol Obstet Invest. 2006;61:223-7.
- [Google Scholar]
- Genital tuberculosis: A major pelvic factor causing infertility in Indian women. Fertil Steril. 1997;67:497-500.
- [Google Scholar]
- Detection of active female genital tuberculosis by molecular method. Int J Pharm Bio Sci. 2010;1:B328-34.
- [Google Scholar]
- Female genital tuberculosis − A retrospective study. Indian J Tuberc. 1998;45:101-3.
- [Google Scholar]
- Cutaneous tuberculosis of the penis and sexual transmission of tuberculosis confirmed by molecular typing. Clin Infect Dis. 2001;33:132-4.
- [Google Scholar]
- Genitourinary tuberculosis: Clinical features in a general hospital population. Am J Med. 1977;63:410-20.
- [Google Scholar]
- Kurman RJ, ed. Genital Tract. Blaustein’s Pathology of the Female (5th). New York: Springer-India; 2002. p. :230.
- Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull. 2005;28:886-92.
- [Google Scholar]
- Potential influence of the microbiome on infertility and assisted reproductive technology. Semin Reprod Med. 2014;32:35-42.
- [Google Scholar]
- Epithelial cytokine responses and mucosal cytokine networks. Trends Microbiol. 1995;3:266-70.
- [Google Scholar]
- Pattern recognition via the toll-like receptor system in the human female genital tract. Mediat Inflamm. 2010;2010:976024.
- [Google Scholar]
- Innate and adaptive immunity in female genital tract: Cellular responses and interactions. Immunol Rev. 2005;206:306-35.
- [Google Scholar]
- Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85-95.
- [Google Scholar]
- Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675-80.
- [Google Scholar]
- Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409-37.
- [Google Scholar]
- Decoding the patterns of self and non-self by the innate immune system. Science. 2002;296:298-300.
- [Google Scholar]
- The evolution of vertebrate toll-like receptors. Proc Natl Acad Sci USA. 2005;102:9577-82.
- [Google Scholar]
- Toll like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428-36.
- [Google Scholar]
- Characterization of toll-like receptors in the female reproductive tract in humans. Hum Reprod. 2005;20:1372-8.
- [Google Scholar]
- Evidence for the presence of toll-like receptor 4 system in the human endometrium. J Clin Endocrinol Metab. 2005;90:548-56.
- [Google Scholar]
- Differential expression of toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect Immun. 2004;72:5799-806.
- [Google Scholar]
- Vaginal commensal bacteria: Interactions with cervix epithelial and monocytic cells and influence on cytokines and secretory leukocyte protease inhibitor (SLPI) In: Thesis printed by Intellecta Infolog. Gothenburg, Sweden: The Sahlgrenska Academy; 2008. [ISBN 978-91-628-7659-3].
- [Google Scholar]
- Menstrual cycle-dependent changes of toll-like receptors in endometrium. Hum Reprod. 2007;22:586-93.
- [Google Scholar]
- SP-A enhances uptake of bacillus Calmette-Guerin by macrophages through a specific SP-A receptor. Am J Physiol. 1997;272:L989-95.
- [Google Scholar]
- Peptidoglycan and lipoteichoic acid-induced cell activation is mediated by toll like receptor 2. J Biol Chem. 1999;274:17406-9.
- [Google Scholar]
- Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96:14459-63.
- [Google Scholar]
- The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766-71.
- [Google Scholar]
- Cutting edge role of toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10-4.
- [Google Scholar]
- Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732-6.
- [Google Scholar]
- Mycobacterium tuberculosis LprA is a lipoprotein agonist of TLR2 that regulates innate immunity and APC function. J Immunol. 2006;177:422-9.
- [Google Scholar]
- Mycobacterium tuberculosis LprG (Rv1411c): A novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J Immunol. 2004;173:2660-8.
- [Google Scholar]
- Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544-7.
- [Google Scholar]
- Regulation of antigen presentation by Mycobacterium tuberculosis: A role for toll-like receptors. Nat Rev Microbiol. 2010;8:296-307.
- [Google Scholar]
- Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J Immunol. 2004;172:4425-34.
- [Google Scholar]
- Different toll-like receptor agonists induce distinct macrophage responses. J Leukoc Biol. 2001;69:1036-44.
- [Google Scholar]
- Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130-5.
- [Google Scholar]
- TLR-2 promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunol Biol. 2008;213:205-24.
- [Google Scholar]
- A family of human receptors structurally related to Drosophila toll. Proc Natl Acad Sci USA. 1998;95:588-93.
- [Google Scholar]
- Inferences, questions and possibilities in toll-like receptor signalling. Nature. 2004;430:257-63.
- [Google Scholar]
- Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987-95.
- [Google Scholar]
- Micrococci and peptidoglycan activate TLR2–>MyD88–>IRAK–>TRAF–>NIK–>IKK–>NF-kappa B signal transduction pathway that induces transcription of interleukin-8. Infect Immun. 2001;69:2270-6.
- [Google Scholar]
- Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev. 2002;15:294-309.
- [Google Scholar]
- Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. 2011;4:271-8.
- [Google Scholar]
- Evolutionary dynamics of human toll-like receptors and their different contributions to host defense. PLoS Genet. 2009;5:e1000562.
- [Google Scholar]
- TLRs mediate IFN-(gamma) production by human uterine NK cells in endometrium. J Immunol. 2006;176:6219-24.
- [Google Scholar]
- Mycobacteria induce IFN-gamma production in human dendritic cells via triggering of TLR2. J Immunol. 2006;176:5173-82.
- [Google Scholar]
- Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development. 2008;135:2001-11.
- [Google Scholar]
- Toll-like receptors in the gonads and reproductive tract: Emerging roles in reproductive physiology and pathology. Immunol Cell Biol. 2007;85:481-9.
- [Google Scholar]
- Inherited disorders of the interleukin-12-interleukin-23/interferon-gamma circuit. In: Hans DO, Smith CI, Puck JM, eds. Primary Immunodeficiency Diseases: A Molecular and Cellular Approach. USA: OUP; 2013. p. :450-65. [Chapter 35]
- [Google Scholar]
- The Arg753Gln polymorphism of the human toll-like receptor 2 gene in tuberculosis disease. Eur Respir J. 2004;23:219-23.
- [Google Scholar]
- Variants in toll-like receptors 2 and 9 influence susceptibility to pulmonary tuberculosis in Caucasians, African-Americans, and West Africans. Hum Genet. 2010;127:65-73.
- [Google Scholar]
- A microsatellite polymorphism in intron 2 of human toll-like receptor 2 gene: Functional implications and racial differences. FEMS Immunol Med Microbiol. 2004;40:163-9.
- [Google Scholar]
- Pathogen specific cytokine release reveals an effect of TLR2 Arg753Gln during Candida sepsis in humans. Cytokine. 2008;41:322-9.
- [Google Scholar]
- Evolution of an intronic microsatellite polymorphism in toll-like receptor 2 among primates. Immunogenetics. 2006;58:740-5.
- [Google Scholar]
- Structural and functional evidence for the role of the TLR2 DD loop in TLR1/TLR2 heterodimerization and signaling. J Biol Chem. 2006;281:30132-42.
- [Google Scholar]
- Toll-like receptor-1, −2 and −6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm Bowel Dis. 2006;12:1-8.
- [Google Scholar]
- Arg753Gln polymorphism of the human toll-like receptor 2 gene from infection to disease in pediatric tuberculosis. Hum Immunol. 2011;72:440-5.
- [Google Scholar]
- IFNG +874T/A, IL10 21082G/A and TNF 2308G/A polymorphisms in association with tuberculosis susceptibility: A meta-analysis study. Hum Genet. 2008;123:477-84.
- [Google Scholar]
- Functional annotations improve the predictive score of human disease-related mutations in proteins. Hum Mutat. 2009;30:1237-44.
- [Google Scholar]
- A single nucleotide polymorphism in the first intron of human interferon-γ gene: Absolute correlation with a polymorphic C/A microsatellite marker of high interferon-γ production. Hum Immunol. 2000;61:863-6.
- [Google Scholar]
- Tuberculosis and HIV: A partnership against the most vulnerable. J Int Assoc Physicians AIDS Care (Chic). 2003;2:106-23.
- [Google Scholar]
- Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739-42.
- [Google Scholar]
- Cutting edge: Toll-like receptor (TLR) 2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol. 2002;169:3480-4.
- [Google Scholar]
- Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS) Nucleic Acids Res. 1989;17:2503-16.
- [Google Scholar]
- Investigation of toll-Like receptor-2 (2258G/A) and interferon gamma (+874T/A) gene polymorphisms among infertile women with female genital tuberculosis. PLoS One. 2015;10:e0130273.
- [Google Scholar]
- Overview of tuberculosis of the female genital tract. J Indian Med Assoc. 1996;94:345-61.
- [Google Scholar]
- Peritoneal tuberculosis simulating advanced ovarian carcinoma: A case report. Gynecol Obstet Fertil. 2003;31:624-6.
- [Google Scholar]
- Neonatal outcome of children born to women with tuberculosis. Med Res. 2001;2:66-99.
- [Google Scholar]
- Tuberculosis in pregnancy − Result of a study in high prevalence area in London. Eur J Obstet Gynecol Reprod Biol. 2006;126:48-55.
- [Google Scholar]
- Laparoscopic findings in female genital tuberculosis. Arch Gynecol Obstet. 2008;278:359-64.
- [Google Scholar]
- Female infertility due to anovulation and defective steroidogenesis in NPC2 deficient mice. Mol Cell Endocrinol. 2010;315:299-307.
- [Google Scholar]
- A cross-sectional study on the current status of female infertility in three counties of Xinjiang Uygur Autonomous Region. Zhonghua Yi Xue Za Zhi. 2011;91:3182-5.
- [Google Scholar]
- Use of endo-ovarian tissue biopsy and pelvic aspirated fluid for the diagnosis of female genital tuberculosis by conventional versus molecular methods. PLoS One. 2014;9:e98005. doi: 10.1371/journal.pone.0098005
- [Google Scholar]
- Postmenopausal tuberculosis endometritis. Infect Dis Obstet Gynecol. 2007;207:27028-30.
- [Google Scholar]
- The prevalence of some bacterial markers in female patients undergoing an initial infertility evaluation in north-east Romania. Roum Arch Microbiol Immunol. 2009;68:171-4.
- [Google Scholar]
- Originalités de la tuberculose dans les pays en voie de développement. Ann Inst Pasteur. 1993;4:208-15.
- [Google Scholar]
- Occurrence of female genital tuberculosis among infertile women: A study from a tertiary maternal health care research centre in South India. Eur J Clin Microbiol Infect Dis. 2014;33:1-12.
- [Google Scholar]
- Overview of clinical tuberculosis. In: Bloom BB, ed. Tuberculosis: Pathogenesis, Protection, and Control. Washington, DC: American Society for Microbiology; 1994. p. :25-46.
- [Google Scholar]
- Convergence of the tuberculosis and diabetes epidemics: Renewal of old acquaintances. Clin Infect Dis. 2007;45:436-8.
- [Google Scholar]
- Vaccines for tuberculosis: Novel concepts and recent progress. Clin Microbiol Rev. 2005;18:687-702.
- [Google Scholar]
- Prevalence and polymorphism in interferon-γ gene (CA) repeats with different stages of endometriosis. Am J Med Biol Res. 2013;1:1-5.
- [Google Scholar]
- Detection of female genital tuberculosis by using endo-ovarian tissue biopsy. Octa J Biosci. 2013;1:98-107.
- [Google Scholar]
- Toll-like receptor gene polymorphism and its relationship with somatic cell concentration and natural bacterial infections of the mammary gland in sheep. Folia Microbiol. 2006;51:647-52.
- [Google Scholar]
- Genetic polymorphism of TLR4 gene and correlation with mastitis in cattle. J Genet Genomics. 2007;34:406-12.
- [Google Scholar]
- Genome-wide association studies of tuberculosis in Asians identify distinct at-risk locus for young tuberculosis. J Hum Genet. 2012;57:363-7.
- [Google Scholar]
- Human genetic susceptibility to intracellular pathogens. Immunol Rev. 2011;240:105-16.
- [Google Scholar]
- Toll-like receptor-2, but not toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol. 2003;171:6187-97.
- [Google Scholar]
- A novel polymorphism in the toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect Immun. 2000;68:6398-401.
- [Google Scholar]
- Recurrent tissue-specific mtDNA mutations are common in humans. PLoS Genet. 2013;9:e1003929.
- [Google Scholar]
- Analysis of somatic mutations in cancer tissues challenges the somatic mutation theory of cancer. eLS 2013 doi: 10.1002/9780470015902.a0024465.
- [Google Scholar]
- Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002;70:4501-9.
- [Google Scholar]
- Hypervirulent M. tuberculosis W/Beijing strains up regulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res. 2005;25:694-701.
- [Google Scholar]
- Association of transforming growth factor-β1 and tumor necrosis factor alpha polymorphisms with anti-SSB/Lα antibody secretion in patients with primary Sjogren’s syndrome. Arthritis Rheum. 2004;50:570-80.
- [Google Scholar]
- Disseminated tuberculosis in IFN-γ gene-disrupted mice. J Exp Med. 1993;178:2243-8.
- [Google Scholar]
- Mutational analysis of interferon-gamma gene in Indian women with female genital tuberculosis. Int J Curr Res Rev. 2012;4:130-40.
- [Google Scholar]
- Familial disseminated atypical mycobacterial infection in early childhood: A human mycobacterial susceptibility gene? Lancet. 1995;345:79-83.
- [Google Scholar]
- An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249-54.
- [Google Scholar]
- Levels of interferon-gamma and tumor necrosis factor-alpha in sera and cervical mucus of fertile and infertile women: Implication in infertility. J Reprod Immunol. 1995;29:105-17.
- [Google Scholar]
- Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145-52.
- [Google Scholar]
- Hypoxia abrogates antichlamydial properties of IFN-γ in human fallopian tube cells in vitro and ex vivo. PNAS. 2010;107:19502-7.
- [Google Scholar]
- Genital tuberculosis and endometriosis coexisting in an infertile female. Indian J Med Spec. 2012;3:91-3.
- [Google Scholar]
- Influence of the interferon-gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) gene polymorphisms in TB occurrence and clinical spectrum. In: Mahboub BH, Vats MG, eds. Tuberculosis − Current Issues in Diagnosis and Management. In Tech; 2013. p. :79-104.
- [Google Scholar]
- Sex differences in the epidemiology of tuberculosis in San Francisco. Int J Tuberc Lung Dis. 2000;4:26-31.
- [Google Scholar]
- Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis. 2007;7:328-37.
- [Google Scholar]
- Comparison of pulmonary and extrapulmonary tuberculosis in Nepal − A hospital-based retrospective study. BMC Infect Dis. 2008;8:1-7. doi: 10.1186/1471-2334- 8-8.
- [Google Scholar]
- A review of sex differences in the epidemiology of tuberculosis. Int J Tuberc Lung Dis. 1998;2:96-104.
- [Google Scholar]
- Extrapulmonary and pulmonary tuberculosis in antananarivo (Madagascar): High clustering rate in female patients. J Clin Microbiol. 2002;40:3964-9.
- [Google Scholar]
- The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N Engl J Med. 1994;330:1703-9.
- [Google Scholar]
- Gender differentials in tuberculosis: The role of socioeconomic and cultural factors. Tuberc Lung Dis. 1996;77:391-400.
- [Google Scholar]
- Attention to gender issues in tuberculosis control. Int J Tuberc Lung Dis. 2001;5:220-4.
- [Google Scholar]
- An integrated epigenetic and genetic approach to common human disease. Trends Genet. 2004;20:350-8.
- [Google Scholar]







