Translate this page into:
To evaluate clinical utility of MUM-1 IHC to detect chronic endometritis due to genital tuberculosis
Address for correspondence: Dr Ashita Punjabi, Department of Reproductive Medicine, Bansal Hospital, Bhopal 462016, Madhya Pradesh, India. E-mail: ashpunjabi@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Punjabi A, Mondal R, Mishra J, Agrawal P, Chittawar PB. To evaluate clinical utility of MUM-1 IHC to detect chronic endometritis due to genital tuberculosis. Fertil Sci Res 2023;10:94-7.
Abstract
Introduction:
Due to its paucibacillary nature, diagnostic tests to detect genital tuberculosis (GTB) suffer from low detection rate. Endometrium is involved in about 50% cases of GTB. Multiple myeloma 1 transcription factor immunohistochemistry (MUM-1 IHC), a marker of plasma cells, has been shown as a sensitive marker for detecting chronic endometritis.
Aim:
The aim of this study is to evaluate the clinical utility of MUM-1 IHC to detect endometrial involvement and chronic endometritis in GTB.
Materials and Methods:
This was a retrospective study conducted in a reproductive medicine unit. Three hundred ninety-one infertile patients who underwent hysterolaparoscopy and in whom MUM-1 IHC was done in the period from May 2019 to December 2020 were included in the study. Diagnosis of GTB was made on laparoscopic findings of tubercles, caseation, dense pelvic adhesions, encysted fluid, tubo-ovarian mass, and cornual blockage in accordance with index tuberculosis (TB) guidelines for extrapulmonary TB in India.
Results:
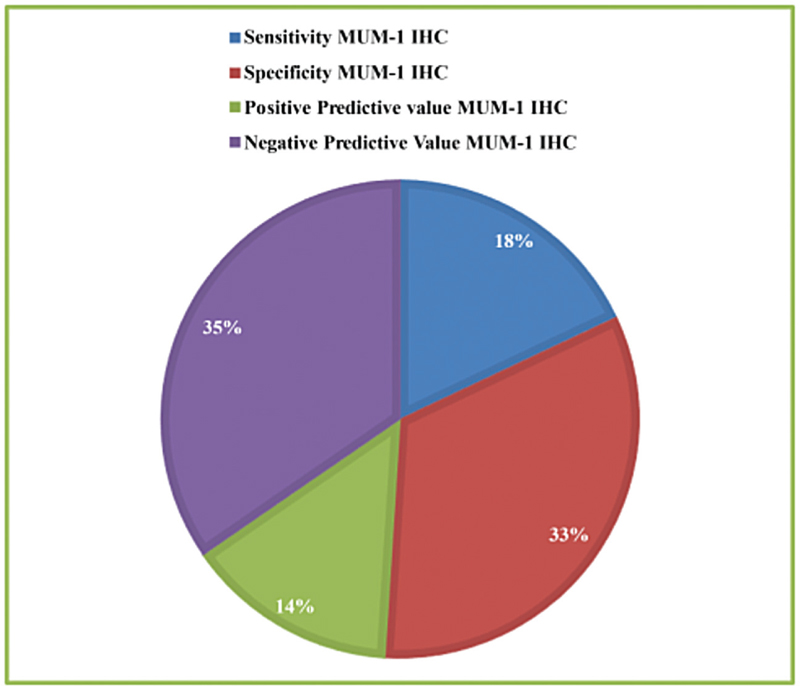
Sixty cases of GTB were diagnosed based on laparoscopic findings. Out of 60 patients, MUM-1 IHC was positive in 28 cases and negative in 32 cases. GeneXpert was negative in all 60 cases of laparoscopy-diagnosed GTB. Histopathology was positive in nine cases of GTB. Sensitivity and specificity of the test are 46.66 and 85.80%, respectively.
Conclusion:
MUM-1 IHC can be proven as a useful marker to rule out endometrial involvement in GTB owing to its high specificity and can be recommended in conditions in which histopathology and GeneXpert are negative; however, prospective studies are needed to confirm the findings of the present study.
Keywords
Genital tuberculosis
Multiple myeloma 1 transcription factor immunohistochemistry
Chronic endometritis
INTRODUCTION
World Health Organization (WHO) in 1993 declared tuberculosis (TB) as a “Global Emergency” considering its significant impact on individual's health and wider social and economic consequences.[1] Mycobacterium tuberculosis is the etiological agent for TB. Female genital tract TB is a leading cause of infertility, especially in developing countries. Being silent in nature, it causes massive destruction of genital structures till it is diagnosed. It is usually secondary to a primary focus elsewhere in the body. The Fallopian tubes are involved in 90% to 100% of cases, endometrium is involved in 50% to 80% of cases, ovaries in 20% to 30%, and cervix is involved in 5% to 15% cases of genital tuberculosis (GTB).[2] Incidence of GTB varies widely ranging from 1% in infertility clinics of USA to 1% to 19% in various parts of India.[2,3,4]
Being paucibacillary in nature, diagnostic tests to evaluate GTB suffer from low detection rate. Culture methods are considered as the gold standard in the detection of GTB. Conventional media such as Löwenstein–Jensen medium requires 1000 bacilli/mL and takes up to 6 to 8 weeks to provide results. Liquid-based culture media such as BACTEC 460 provide results within 2 weeks and provide rapid assessment for drug sensitivity patterns.[5] Polymerase chain reaction (PCR) is a rapid, sensitive, and specific method that can detect <10 bacilli/mL of the sample and results are available within 1 to 2 days,[6] but it suffers from high false positive and false negative rates. Laparoscopy involves operative and anesthetic risks and risk of infection [Figure 1].
- Sensitivity and Specificity of MUM1-IHC.
CD 138 is widely used as an ancillary immunohistochemistry stain to identify plasma cells; however, it has a drawback of high background reaction. Multiple myeloma 1 transcription factor immunohistochemistry (MUM-1 IHC) has been shown to be a more sensitive stain than CD 138 in diagnosing chronic endometritis in addition to having clean background.[7]
Since endometrium is involved in almost half of the cases of genital TB, we sought to evaluate MUM-1 as a marker of endometrial involvement and chronic endometritis due to GTB.
MATERIALS AND METHODS
Ethical Clearance
The study was conducted after taking ethical clearance from the Institutional Human Ethics Committee, Bansal Hospital, Bhopal, Madhya Pradesh (BH006).
This was a retrospective study conducted in a Reproductive Medicine Unit. Three hundred ninety-one infertile patients who underwent hysterolaparoscopy and in whom MUM-1 IHC was done in the period from May 2019 to December 2020 were included in the study.
The primary outcome measure of the study was to evaluate MUM-1 IHC as a marker of endometrial involvement and chronic endometritis due to GTB. The cases were diagnosed on the basis of definite laparoscopic findings such as tubercles, caseous nodules, or probable findings such as dense pelvic adhesions, peritoneal or perihepatic adhesions, encysted fluid, tubo-ovarian mass, congested or convoluted tubes, and cornual blockage.[8]
Following hysterolaparoscopy, endometrial samples were sent for MUM-1 IHC and cartridge-based nucleic acid amplification test (CB-NAAT) in normal saline and histopathological examination in formalin.
For MUM-1 IHC, ready to use monoclonal mouse antibody is used to label formalin fixed, paraffin-embedded tissue sections. The staining steps and incubation times are programmed into the software of DAKO Autostainer. Cells labeled by the antibody display predominantly nuclear staining, although weak to moderate staining of the cytoplasm is also present in most cases with nuclear staining.
A value of “1” was assigned to a positive stain if at least one plasma cell had been observed. A value of “0” was given to a negative stain if no plasma cell was detected in the respective endometrial tissue section value.[7]
Statistical Analysis
For continuous data, the descriptive statistics was used such as mean and standard deviation. Frequency and percentage were used for categorical data. Microsoft Office Excel 97 to 2003 was used for tabulation of data and statistical analysis.
RESULTS
Mean age of women was 32.24 ± 0.26 years. Sixty cases of GTB were diagnosed based on hysterolaparoscopic findings. Out of 60 patients, MUM-1 IHC was positive in 28 cases and negative in 32 cases [Table 1]. GeneXpert was negative in all 60 cases of laparoscopy-diagnosed GTB. Histopathology was positive in nine cases of GTB.
| MUM-1 IHC Positive | MUM-1 IHC Negative | |
|---|---|---|
| GTB cases | 28 | 32 |
| Non-GTB cases | 47 | 284 |
| Sensitivity MUM-1 IHC | 46.66% | |
| Specificity MUM-1 IHC | 85.80% | |
| Positive predictive value MUM-1 IHC | 37.33% | |
| Negative predictive value MUM-1 IHC | 89.87% |
GTB = genital tuberculosis, MUM-1 IHC = multiple myeloma 1 transcription factor immunohistochemistry
DISCUSSION
Diagnosis of GTB poses a great challenge owing to its paucibacillary nature and varied clinical features. Culture is considered as a gold standard but paucibacillary nature of GTB can lead to negative culture. In study by Sharma et al., adhesions were present in 88.8% women. Other findings were beading of tubes, presence of tubercles, caseation, and tubo-ovarian masses.[9] The index TB guidelines on extrapulmonary TB in India guide for diagnosing GTB based on laparoscopic appearance typical for TB.[10] In the present study, cases of GTB were diagnosed on laparoscopy.
Delay in treatment can cause significant morbidity and can have serious repercussions on the fertility of women. Endometrium is involved in almost half of the cases of GTB. Endometrial involvement can destroy endometrium causing Asherman syndrome. Therefore, there is a need to evaluate diagnostic modalities, which can diagnose endometrial involvement in GTB so treatment can be started at the earliest to prevent progression of the disease.
MUM-1 is a lymphocyte-specific transcriptional factor, a member of interferon regulatory factor (IRF) family. It contributes to the regulation of immunoglobin gene expression in the final step (late centrocyte) of B-cell differentiation within germinal center. MUM1 is not only a marker for plasma cells, but also highlights activated B and T cells; or only lymphoid cells in an activated state.[11] Physiologically, B lymphocytes tend to aggregate in the basalis layer and constitute approximately 1% of the normal lymphoid population. In endometritis, the B cells increase in the endometrium aggregating in the functionalis rather than the basalis layer with no significant change in the T-cell population.[12][13] Therefore, the presence of the MUM1-postive lymphoid B cells in the endometrial stroma may be a clue for the presence of active inflammation versus normal or physiological conditions.[7] Recently, a new concept of impaired inflammatory state of endometrium has emerged instead of chronic endometritis, which considers both infectious factors (Streptococcus, Escherichia coli, Enterococcus, GTB, etc.) and noninfectious factors (endometriosis, adenomyosis, endometrial polyp, autoimmune, and hormonal disturbances) unlike chronic endometritis which only refers to infectious pathology.[14]
To our knowledge, the present study is the first study to evaluate MUM-1 IHC as marker of endometrial involvement and chronic endometritis in GTB. Sensitivity and specificity of the test are 46.66% and 85.80% respectively. Sensitivity of histopatholgy is 15% and specificity is 100% in our study, whereas GeneXpert has 0% sensitivity and 99.3% specificity [Table 2]. Garg et al.[15] did not find any patient positive with GeneXpert among 81 infertile women suspected of having GTB as a cause of infertility.[15] MUM-1 has a good negative predictive value along with hysterolaparoscopy in ruling out endometrial involvement and chronic endometritis due to TB.
| MUM-1 IHC (%) | Histopathology (%) | GeneXpert (%) | Chi-square | P value | |
|---|---|---|---|---|---|
| Sensitivity | 46.66 | 15 | 0 | 34.538 | 0.000000004 |
| Specificity | 85.80 | 100 | 99.39 | ||
| Positive predictive value | 37.33 | 100 | 0 | ||
| Negative predictive value | 89.87 | 86.64 | 84.57 |
Limitations of the study include its being retrospective in nature which could have introduced bias in the study and small sample size.
CONCLUSION
MUM-1 IHC can be proven as a useful marker to rule out endometrial involvement in GTB owing to its high specificity and can be recommended in conditions in which histopathology and GeneXpert are negative; however, prospective studies are needed to confirm the findings of the present study.
Financial support and sponsorship
There has been no financial vested interest.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Genital tuberculosis—a major pelvic factor causing infertility in Indian women. Fertil Steril. 1997;67:497-500.
- [CrossRef] [PubMed] [Google Scholar]
- Genital tuberculosis in Indian infertility patients. Int J Gynecol Obstet. 2007;97:135-8.
- [CrossRef] [PubMed] [Google Scholar]
- Management of female genital tuberculosis: re-appraised. Obstet Gynaec Today. 2006;11:506-9.
- [Google Scholar]
- Multiple myeloma 1 transcription factor is superior to CD138 as a marker of plasma cells in endometrium. Int J Surg Pathol. 2019;27:372-9.
- [CrossRef] [PubMed] [Google Scholar]
- Genital TB-diagnostic algorithm and treatment. Indian J Tuberc. 2020;67:S111-8.
- [CrossRef] [PubMed] [Google Scholar]
- Genital tuberculosis: an important cause of Asherman's syndrome in India. Arch Gynecol Obstet. 2008;277:37-41.
- [CrossRef] [PubMed] [Google Scholar]
- Index-TB guidelines: guidelines on extrapulmonary tuberculosis for India. Indian J Med Res. 2017;145:448-63.
- [Google Scholar]
- A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood. 2000;95:2084-92.
- [CrossRef] [PubMed] [Google Scholar]
- Benign diseases of the endometrium. In: Kurman RJ, Ellenson LH, Ronnett BM, eds. Blaustein's Pathology of the Female Genital Tract. New York, NY: Springer. 2011;307:58.
- [CrossRef] [Google Scholar]
- Immunohistochemical characterization of endometrial leucocytes in endometritis. Histopathology. 2004;45:625-32.
- [CrossRef] [PubMed] [Google Scholar]
- Impaired inflammatory state of the endometrium: a multifaceted approach to endometrial inflammation. Current insights and future directions. Prz Menopauzalny. 2020;19:90-100.
- [CrossRef] [PubMed] [Google Scholar]
- GeneXpert test and endometrial histological findings in infertile women. Int J Reprod Contracept Obstet Gynaecol. 2018;7:1480-3.
- [CrossRef] [Google Scholar]







