Translate this page into:
Fertility Problems Due to Chrono-Disruption: A Mini Review

*Corresponding author: Prof. Chandana Haldar, MSc, PhD, Department of Zoology, Banaras Hindu University, Varanasi, India chaldarbhu@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Das M, Yadav SK, Mishra NK, Haldar C. Fertility Problems Due to Chronodisruption: A Mini Review. Fertil Sci Res. 2024;11:13. doi: 10.25259/FSR_11_2024
Abstract
Circadian rhythm coordinates many physiological and behavioural processes and is under the control of the endogenous suprachiasmatic nucleus (SCN) entrained by a light-dark cycle. The core clock genes, Bmal1, Clock, Per, and Cry, organised are organised in a transcriptional–translational feedback loop (TTFL) of the 24-h cycle. Recently, these clocks have been reported in the female reproductive organs, i.e., ovary, uterus, etc. The presence of clock genes in female reproductive tissues has generated the interest of reproductive biologists towards chrono-regulation, as the clock genes may play a central role in coordinating the circadian rhythm of the reproductive process, i.e., form ovulation, fertilisation and implantation to lactation. Studies have demonstrated a critical connection between disturbance of the master circadian clock (SCN) of the hypothalamus and infertility.
Artificial lighting is a worldwide increasing problem in the physiology of many vertebrates controlled by circadian/seasonal entrainments. The effect of artificial light on circadian dysregulation in the night-shift working females resulted in severe reproductive dysfunction (low pregnancy outcomes, childbirth weight, multiple miscarriages, etc.). Inappropriate exposure to artificial light/chrono disruption can lead to several disturbances to the biological/physiological rhythms of metabolomics and hormones.
In the present mini review, we proposed that the duration of light affects the circadian coordination of central (SCN) and peripheral clock (ovarian and uterine clock) oscillations regulating uterine tissue homeostasis and pregnancy success in golden hamsters. Two major aspects of light exposure, i.e., (1) continuous and (2) long-time artificial light at night (ALAN), influence circadian oscillations of clock genes on SCN, ovary, and uterus as well as the molecular mechanism behind the altered clock entrainments that finally induce the female reproductive impairments via alteration in hypothalamic-pituitary-gonadal (HPG) axis.Keywords: Smoking, Semen Quality, Sperm Parameters, Male Infertility, North India, Fertility Treatment, Smoking Cessation.
Keywords
Chronodisruption
Artificial light
Clock genes
Female infertility
Circadian
INTRODUCTION
Circadian Clock – SCN
The circadian clock, or the suprachiasmatic nucleus (SCN), is a molecular timekeeping machine located in the anterior hypothalamus at the dorsal side of the optic chiasma.[1] SCN is the central circadian pacemaker consisting of a cluster of 20,000 excitatory neurones and astrocytes, whose activities fluctuate with the 24-h cycle. It participates in diverse photic (light) and non-photic (metabolic, temperature) stimuli for the regulation of physiological activities, which include the sleep/wake cycle, energy metabolism, hormonal regulation, immune functions, and cell proliferation.[2] Retinal light information from the retinohypothalamic tract (RHT) reaches the SCN and communicates periodically with non-SCN secondary brain clocks and then with peripheral organ clocks (e.g., heart, liver, kidney, ovary, uterus, adrenal, etc.) to synchronise with external day and night cycles.[2] This master clock, SCN, rhythmically coordinates organismal physiology and behaviour in response to daily environmental changes by synchronising non-SCN subordinate brain clocks and peripheral organ clocks by neural (neurotransmitters and neuropeptides) and humoral outputs (hormonal secretion, neural innervations, and autonomic nervous system).[3]
CIRCADIAN CONTROL ON FEMALE FERTILITY
Circadian Rhythm and Ovulation
Ovulation is the interplay of various hormones that is crucial to making ovulation successful. The circadian system regulates the HPG axis, which in turn plays a prominent role in the management of steroid hormone synthesis, follicular development and ovulation till maturation.[4,5] Successful pregnancy commences with the release of mature oocyte from the ovary and terminates with parturition. Rhythmical expression of kisspeptin (Kiss1) activates the gonadotropin-releasing hormone (GnRH) neurons.[6] At the mature pre-ovulatory stage, the GnRH secretion becomes stimulatory and simultaneously excites the anterior pituitary to release LH, leading to the discharge of mature oocytes from the ovary. A wide array of studies regarding the circadian regulation of female reproduction is confined to the hormonal crosstalk between SCN and LH surge.[7]
To understand the role of clock genes on the ovulation process, an assessment was conducted by the researchers, where the expression of aryl hydrocarbon receptor nuclear translocator-like protein (Arntl) or Bmal1 and Per2 was checked over 2 days during the oestrous cycle, which suggested that the rhythm of circadian clock genes of the ovary might be regulated significantly by LH secretion.[8] Reports also suggested that in granulosa cells (GCs) several genes are clock-controlled such as LH receptor (Lhcgr), prostaglandin synthesis enzymes (Ptgs2), steroidogenic enzymes (e.g., Cyp11a1, aromatase, etc.), delta-aminolevulinate synthase 1 (Alas1), Ppary coactivator 1 alpha (Pparyc1a), interleukin 6 (IL6), and gap junction protein Connexin-43 (Cx43) that are important in follicular development and ovulation.[9] It has also been stated that the clock gene-altered estradiol signalling.[10] Further, the promoter region of cyclooxygenase-2 (COX2), a rate-limiting enzyme for prostaglandin synthesis, has an E-box sequence which binds to the CLOCK: BMAL1 heterodimers and activates its rhythmic transcription during follicular maturation.[7] Further, this rhythmic accumulation of COX2 enzyme regulates the rhythmic synthesis of prostaglandin E2 (PGE2) and prostaglandin F2a (PGF2a), resulting in increased levels of prostaglandin synthesis.[7]
Circadian Rhythm and Fertilization
The fertile window initiates approximately between 3 and 6 days before LH and remains till the deposited sperms remain viable. After 12–48 h of the LH surge, ovulation occurs.[11,12] It has been observed that for successful reproductive outcomes, mating should occur early, just after ovulation.[13] As the level of estradiol increases by 2–3 days before the LH surge, the mucus layer of the reproductive tract becomes permeable due to an increase in hydration, thus allowing sperms to travel into the uterus.[14]
Subsequently, the timing of LH surge and estradiol level creates an important milieu for sperm motility and fertilisation capability.[11] It was well established that clock genes are responsible for estradiol synthesis and LH surge; thus, it can be proposed that the fertilisation capacity is also indirectly regulated by circadian timing.[11] Studies with hypophysectomised juvenile rats confirmed no such circadian rhythm pattern of Arntl and Per2 due to the absence of LH surge. Further, in mice, a lack of both Cry1 and Cry2 was noted in the absence of LH surge.[8,15–17] Therefore, hormonal coordination with clock gene oscillations is important for ovulation and fertilisation.
Circadian Rhythm and Implantation
Implantation is a process of adherence of the blastocyst to the receptive endometrium, which includes the series of changes in the uterine environment guided by ovarian E2 and P4 to establish mutual signalling between the blastocyst and the uterus.[18,19] During the period of uterine receptivity, the clock genes and their proteins express rhythmically within the uterine epithelium, stroma, and myometrium.[20–22] It has been reported that ovarian steroid hormones have the capability to change the expression of clock genes.[23] Progesterone not only increases the expression of neuronal PAS domain protein 2 (Npas2), Clock, Cry1, and Per1 expression but also decreases Rev-erbβ and retinoic acid receptor-related orphan receptor-a (RORg) mRNA expression.[24] Additionally, over the peri-implantation period, the vascular endothelial growth factor (VEGF) mRNA (an E-box containing mRNA at its promoter) also expresses rhythmically in the uterus.[20] A knockout study showed that implantation failure in Bmal1 knockout mice is due to a lack of P4 biosynthesis enzyme expression. In the luteinised GCs, Per1 and Per2 mutant mice showed 80% fertility.[22, 25, 26] Studies confirmed that women with polymorphic Bmal1, Bmal2, Clock, and Npas2 express normal female reproductive characteristics.[27] The report also suggested that the Bmal1 gene (rs2278749 TT) contains SNP in association with an increased number of pregnancies as well as an increased number of miscarriages. Interestingly, they also revealed that Npas2 rs11673746 T carriers showed lower miscarriages.[27] Hence, it can be concluded that implantation is also crucially governed by the circadian clock system.
Clock Rhythm and Embryogenesis
Reports suggest clock genes’ rhythmic expression in unfertilised oocytes, pronuclear zygotes, blastocysts, and embryos.[28] Further, CLOCK expression was noted at a high level throughout the pregnancy, starting from fertilisation. Studies have established that the incidence of all core clock genes mRNA expression occurs at various stages of oocytes and pre-implantation development of mouse and rabbit embryos.[29] The uterus, placenta, and membrane of day 16 (D16) embryos were reported to have a stout rhythm of clock genes.[24] Per3 has also been reported in association with corticogenesis, in which PER3 regulates excitatory neurone migration and synaptic network formation.[30] An in vitro study of human embryonic stem cells (ESCs) showed that all clock genes are expressed in embryonic stem cells but not in a rhythmic way; thus, clock genes possess an important role in embryogenesis.[31]
Circadian Rhythm and Gestation
Studies have revealed that the circadian timing of birth depends not only on the master clock (SCN) but also on the peripheral clocks. These clock genes participate in several reproductive peripheral tissues at the time of gestation, revealing that circadian rhythmicity associated with clock genes is important for parturition.[32] The SCN lesion in rats showed a single peak in parturition frequency while in normal conditions, rats delivered during a 36-h time window with two peaks in parturition frequency 24 h apart.[32] Clock-/- study revealed that pregnant mice lacking the Clock genes not only showed oa high incidence of foetal resorption but also had prolonged and unproductive labour.[33,34] Some case studies have revealed that night time-delivered mice lack Bmal1 in the myometrium.[35] These reports suggested that clock gene oscillation is essential for healthy labour.
Circadian Rhythm and Female Hormonal Control
LH and FSH
The master clock communicates with several peripheral clocks via various but poorly defined connections of neural and humoral centres. After receiving the environmental signal, the core clock genes of SCN (Bmal, Clock, Per, and Cry) send the timing information via efferent neurones to the other part of the brain, mainly paraventricular nucleus (PVN), medial pre-optic area, dorsomedial nucleolus of hypothalamus, and the pineal gland (MEL), and regulate the secretion of hypophyseal hormones and melatonin.[36] SCN is reported to synchronise the GnRH neurones in the medial preoptic area (MPOA).[37]
Estrogen (E2) & progesterone (P4)
E2 and P4 of the HPG axis are mainly responsible for all the molecular events related to pregnancy, such as ovulation, fertilisation, uterine receptivity, implantation, decidualisation, gestation, and parturition.[38] Steroids influence the phase, amplitude, and period of circadian clock gene rhythms in SCN.[38] The estrogen receptors (ERa, ERβ) possess direct links with core clock genes.[39] The promoter of ERβ covers an evolutionary conserved E-box, which is the binding site of CLOCK/BMAL1 heterodimer in an arhythmic manner and regulates the rhythmic expression of the receptor.[40,41] A complex loop relationship between estradiol signalling and clock proteins exists where estradiol may impact the core clock machinery.
HPA Axis
The HPA axis manifests all the maternal-foetal physiological adaptations for a successful pregnancy. On the contrary, the high foetal demand are for energy and the maternal physiological stress management are fulfilled by the HPA axis adaptation, which in turn promotes the release of the energy store as well as regulates maternal-foetal immune parameters.[42,43] Moreover, reports suggested that in the SCN, clock genes express rhythmic controls on the circadian variations of both the HPA axis and the respective gonadal and adrenal peripheral clocks.[44] Literature suggests that balance in sleep and wakefulness preserves both the body clock and a good melatonin level that keeps the body away from chrono-disruption-related diseases. The work at night/shift work increases the time/duration of light exposure at night, consequently suppressing melatonin production, hence, potentially elevating cancer risk and the substantial downstream molecular mechanisms of the circadian clock genes with melatonin.
The destruction of the master clock through any environmental or lifestyle shifts may abolish the peripheral clock synchronisation and disrupt maternal physiological adjustments during pregnancy. We provided the fundamental explanation of the much-required anthropological perspective of the hazardous effects of light, and it may expand our understanding of the duration and spectral composition of light is essential for coordination between central and peripheral clock genes to regulate female reproductive health, including pregnancy.
Concept of chronodisruption
In 2003, the term “chronodisruption” was first invented by T. C. Erren, R. J. Reiter, and C. Piekarski of the University of Cologne.[45] Previously, desynchronisation 24-, there was a concept of “chronodisturbance” (or absence of adverse consequences for health), “circadian disrupt", or “disruption of circadian rhythm,” suggesting the desynchronisation of 24-h rhythms on adverse health. Chronodisruption was further classified as “exogenous and endogenous exposures which can disrupt the timing and order of physiologic functions."[45] The nighttime use of artificial light and backlit screens during the night is a very prominent example of a chronodisrupter.[46] The International Agency for Research on Cancer 2007 classified shift work as a chronodisrupter and also as a probable human carcinogen, and since then, “chronodisruption” has drawn the attention of the scientific world [Figure 1].[46]
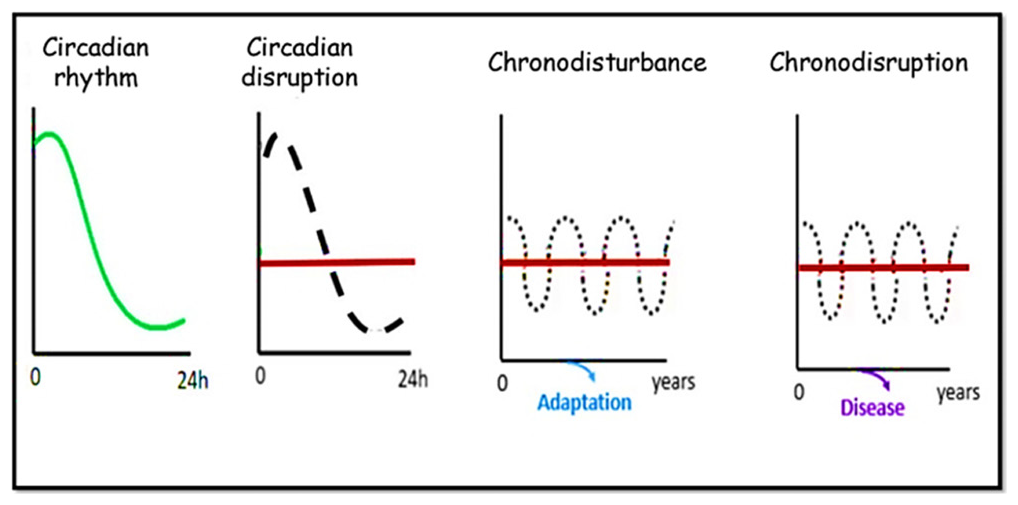
- Concept of circadian disruption, chronodisturbance and chronodisruption (Modified from https://doi.org/10.3390/toxins12030151).
Light at Night Induced Chronodisruption and Female Fertility
In humans, it has been reported that circadian disruption can possess deleterious effects on female reproduction that are linked to female hormonal systems, resulting in reproductive dysfunction-related subfertility.[47] Studies have suggested the presence of multiple targets of the molecular loop of the circadian clock that is associated with important physiological functions of the hypothalamus-pituitary-gonadal axis, resulting in disturbed female reproduction.[48] Previously, survey studies on hospital nurses showed that night shift or rotating shift nurses experience painful and irregular menstruation with changes in their cycle length, duration of bleeding period, menstrual flow, and dysmenorrhea.[49,50] Same group of researchers showed that hospital nurses in shift work had irregular menstrual cycles than permanent day shift working nurses.[49,50] The expansion of the menstrual cycle is connected to the length of the follicular phase. These results suggest that rotating shift work may induce a delay in ovulation.[50] Further, studies on knockout or transgenic animal models have shown that clock gene synchronisation is critical for reproductive success in female rodents. Circadian dysregulation in clock genes is also known to disrupt pregnancy, such as time of gestation, circadian timing of birth, etc. [Figure 2].[48,51]
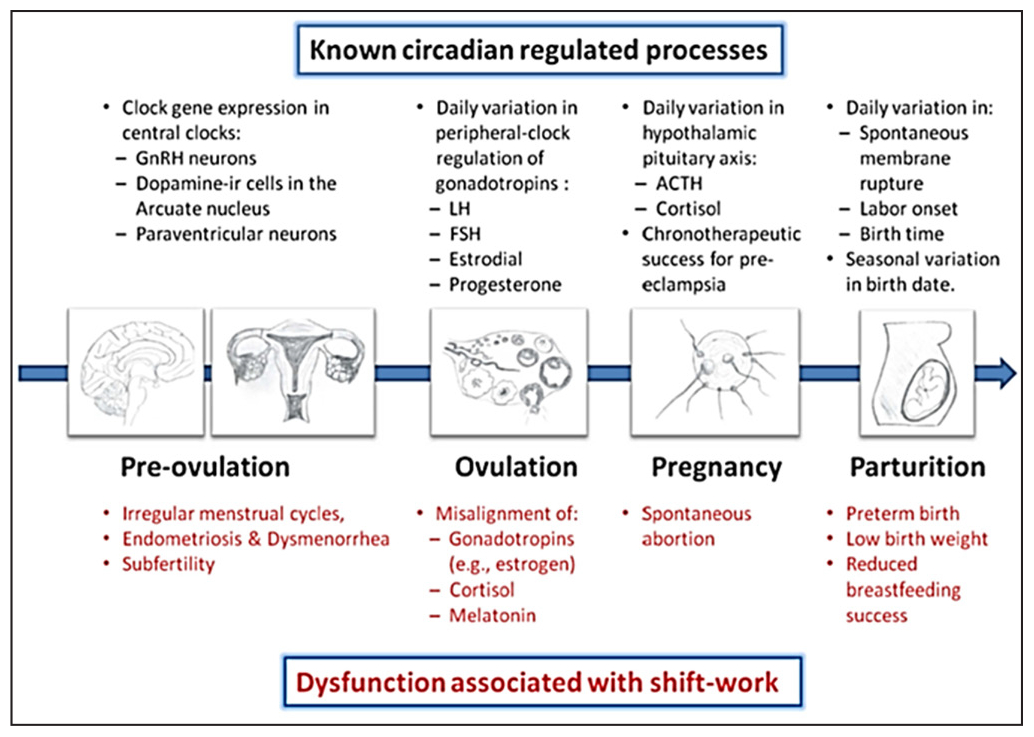
- Reproductive impairment associated with shift work. LH: Luteinizing hormone, FSH: Follicle Stimulating hormone, ACTH: Adrenocorticotropic hormone. (Modified from https://doi.org/10.3389/fendo.2013.00092).
The biological rhythms of humans are reported to be affected by constant light.[52] Several studies have revealed that the biological crosstalk between the mother and foetus is affected by constant light. Therefore, disturbances in the ratio of light and dark not only disturb the pattern of sleep in such as the mother but also affect the early stages of various development, such as visual development of the foetus, postnatal weight gain, etc.[53–57]
Our studies with mice suggested that in reproductive tissue, continuous light (LL) damages the circadian coordination between central and clock genes, leading to disturbed uterine physiology and, thus, altered pregnancy. Studies on pregnant mice revealed lowered progesterone under ;the LL condition; mice under the LL condition downregulated expression of progesterone receptor (PR) and PR-dependent uterine Homeobox A-10 (Hoxa10) proteins. It lowered pregnancy outcomes in LL mice. Hence, we may propose that in females, the duration of light exposure at the workplace/home is important to minimise pregnancy-related problems [Figure 3].[58]
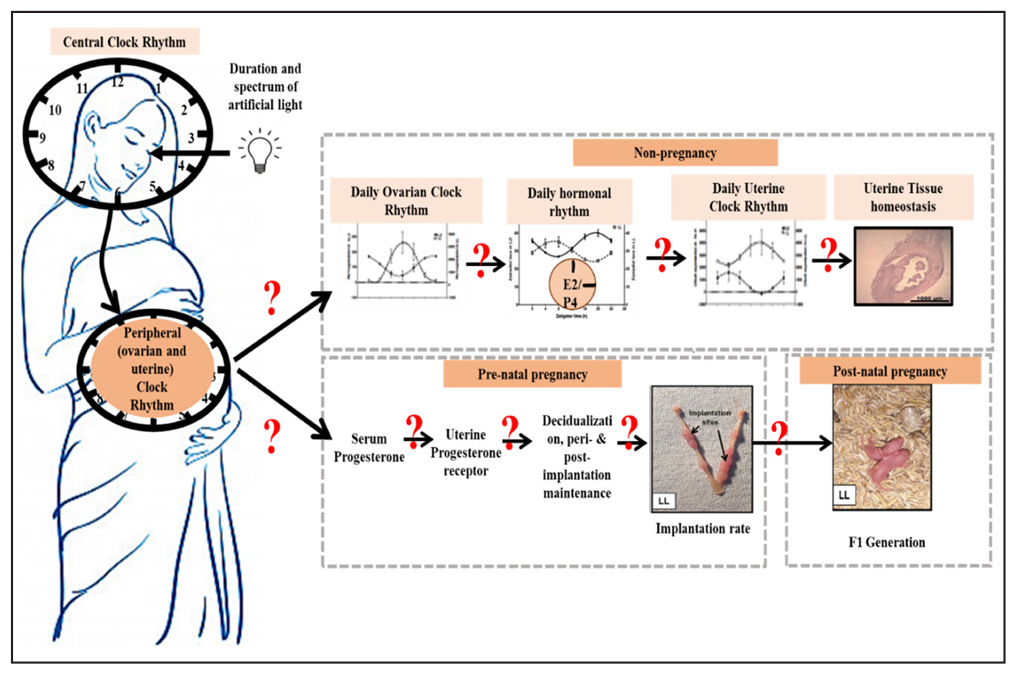
- Showing the effect of duration of light in desynchronization of central clock and clocks in reproductive organs (Modified from https://doi.org/10.1007/s43630-022-00210-6).
LL is always negatively affecting the reproductive health of females. LL exposure increases the endometrium thickness and reduces the myometrium, as the condition of uterine adenomyosis. LL alters the expressions of clock genes in SCN, ovary, and uterus along with serum estradiol rhythm gets disturbed as well in non-pregnant females Egf gets upregulated, and Aanat, Cx26, and Cx43 mRNA levels get downregulated in the uterus. LL exposure desynchronises the central and peripheral reproductive clock and thus uterine physiology via the Akt/FoxO1 pathway, as reported in Golden Hamsters.[59] Hence, LL could be a risk factor for female fertility. Thus, the duration of the light is important in considering the physiological consequences of female reproductive abnormalities.
CONCLUSION
Our review may provide a fundamental explanation of the much-required anthropological perspective of the hazardous effects of light. It may expand our understanding of the duration and spectral composition of light that are essential in coordination between central and peripheral clock genes in the regulation of female reproductive health and pregnancy success that minimises the risk factor for females at the workplace/home. ALAN is unavoidable in the current scenario. Not only streetlights, but the use of electronic devices is increasing day by day which also emits blue-wavelength-rich light. To find out the future possibilities to restore a healthy pregnancy in the present urbanised world, it is necessary to understand the cause and the mechanism involved in the prevention of normal physiological processes of pregnancy. However, the information is still not enough regarding the spectrum and intensity of light on the clock regulation of pregnancy impairments like unsuccessful implantation/spontaneous abortions. More studies are required to delineate the molecular mechanism of clock genes and the effect of light/light spectrum on chronodisruptive female fertility problems.
Acknowledgement
The authors would like to acknowledge the ICMR ad-hoc project (P-14/267) and Institute of Eminence (IoE) research grant, BHU, to Dr. Sanjeev K. Yadav.
Author contribution
Conception and design: MD, SKY, Collection and assembly of data: MD, NKM, Writing, review and editing: MD, SKY, CH, Final approval of manuscript: All authors.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patients consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
The authors are thankful to the Indian Council of Medical Research and Institute of Eminence, BHU, for financial support to Dr. Sanjeev K. Yadav.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Communicating Clocks Shape Circadian Homeostasis. Science.. 2021;371((6530)):eabd0951.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The Suprachiasmatic Nucleus: A Responsive Clock Regulating Homeostasis by Daily Changing the Setpoints of Physiological Parameters. Auton Neurosci.. 2019;218:43-50.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of the Clock Genes Per1 and Bmal1 During Follicle Development in the Rat Ovary. Effects of Gonadotropin Stimulation and Hypophysectomy. Cell Tissue Res.. 2012;350((3)):539-48.
- [CrossRef] [PubMed] [Google Scholar]
- Timing of the Ovarian Circadian Clock is Regulated by Gonadotropins. Endocrinology.. 2009;150((9)):4338-47.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The Role of Kisspeptin in Female Reproduction. Int J Endocrinol Metab.. 2017;15((3)):e44337.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Circadian Clocks in the Ovary. Trends Endocrinol Metab.. 2010;21((10)):628-36.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Circadian Clock Gene Expression in the Ovary: Effects of Luteinizing Hormone. Biol Reprod.. 2006;75((4)):624-32.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of Clock-Controlled Genes in Mammals. PLoS One.. 2009;4((3)):e4882.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The Interplay Between Circadian System, Cholesterol Synthesis, and Steroidogenesis Affects Various Aspects of Female Reproduction. Front Endocrinol (Lausanne).. 2013;4:111.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Application of a Method for Estimating Day of Ovulation Using Urinary Estrogen and Progesterone Metabolites. Epidemiology.. 1995;6((5)):547-50.
- [CrossRef] [PubMed] [Google Scholar]
- Temporal Relationship and Reliability of the Clinical, Hormonal, and Ultrasonographic Indices of Ovulation in Infertile Women. Obstet Gynecol.. 1990;75((3 Pt 1)):412-6.
- [PubMed] [Google Scholar]
- Synchronization of the Ovulation and Copulation Timings Increased the Number of in Vivo Fertilized Oocytes in Superovulated Female Mice. PLoS One.. 2023;18((2)):e0281330.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Analysis of Pre-Ovulatory Changes in Cervical Mucus Hydration and Sperm Penetrability. Adv Contracept.. 1997;13((2–3)):143-51.
- [CrossRef] [PubMed] [Google Scholar]
- Interacting Molecular Loops in the Mammalian Circadian Clock. Science.. 2000;288((5468)):1013-9.
- [CrossRef] [PubMed] [Google Scholar]
- Photic Induction of mPer1 and mPer2 in Cry-Deficient Mice Lacking a Biological Clock. Science.. 1999;286((5449)):2531-4.
- [CrossRef] [PubMed] [Google Scholar]
- Differential Regulation of Mammalian Period Genes and Circadian Rhythmicity by Cryptochromes 1 and 2. Proc Natl Acad Sci USA.. 1999;96((21)):12114-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Physiological and molecular determinants of embryo implantation. Mol Aspects Med.. 2013;34((5)):939-80.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Molecular Cues to Implantation. Endocr Rev.. 2004;25((3)):341-73.
- [CrossRef] [PubMed] [Google Scholar]
- Down-Regulation of Circadian Clock Gene Period 2 in Uterine Endometrial Stromal Cells of Pregnant Rats During Decidualization. Chronobiol Int.. 2011;28((1)):1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of the Estrous Cycle on Clock Gene Expression in Reproductive Tissues: Effects of Fluctuating Ovarian Steroid Hormone Levels. Steroids.. 2010;75((3)):203-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clock Gene Expression in Gravid Uterus and Extraembryonic Tissues During Late Gestation in the Mouse. Reprod Fertil Dev.. 2010;22((5)):743-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Up-Regulation of Per1 Expression by Estradiol and Progesterone in the Rat Uterus. J Endocrinol.. 2007;194((3)):511-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cryptochrome and Period Proteins are Regulated by the CLOCK/BMAL1 Gene: Crosstalk Between the PPARs/RXRalpha-Regulated and CLOCK/BMAL1-Regulated Systems. PPAR Res.. 2008;2008:348610.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Low Reproductive Success in Per1 and Per2 Mutant Mouse Females Due to Accelerated Ageing? Reproduction.. 2008;135((4)):559-68.
- [CrossRef] [PubMed] [Google Scholar]
- Nonredundant Roles of the mPer1 and the mPer1 and mPer2 Genes in the Mammalian Circadian Clock. Cell.. 2001;105((5)):683-94.
- [CrossRef] [PubMed] [Google Scholar]
- ARNTL (BMAL1) and NPAS2 Gene Variants Contribute to Fertility and Seasonality. PLoS One.. 2010;5:10007.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Circadian Clockwork Genes are Expressed in the Reproductive Tract and Conceptus of the Early Pregnant Mouse. Reprod Biomed Online.. 2002;4((2)):140-5.
- [CrossRef] [PubMed] [Google Scholar]
- Expression Analysis of Circadian Genes in Oocytes and Preimplantation Embryos of Cattle and Rabbits. Anim Reprod Sci.. 2010;121((3–4)):225-35.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Per3, A Circadian Clock Gene, in Embryonic Development of Mouse Cerebral Cortex. Sci Rep.. 2019;9((1)):5874.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Circadian Networks in Human Embryonic Stem Cell-Derived Cardiomyocytes. EMBO Rep.. 2017;18((7)):1199-212.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The Circadian-Gated Timing of Birth in Rats: Disruption by Maternal SCN Lesions or by Removal of the Fetal Brain. Brain Res.. 1987;403((2)):398-402.
- [CrossRef] [PubMed] [Google Scholar]
- Developmental and Reproductive Performance in Circadian Mutant Mice. Hum Reprod.. 2006;21((1)):68-79.
- [CrossRef] [PubMed] [Google Scholar]
- Circadian Clock Mutation Disrupts Estrous Cyclicity and Maintenance of Pregnancy. Curr Biol.. 2004;14((15)):1367-73.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Generation of Myometrium-Specific Bmal1 Knockout Mice for Parturition Analysis. Reprod Fertil Dev.. 2012;24((5)):759-67.
- [CrossRef] [PubMed] [Google Scholar]
- The Genetics of Mammalian Circadian Order and Disorder: Implications for Physiology and Disease. Nat Rev Genet.. 2008;9((10)):764-75.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kisspeptins and GnRH Neuronal Signalling. Trends Endocrinol Metab.. 2009;20((3)):115-21.
- [CrossRef] [PubMed] [Google Scholar]
- Clock Genes Alterations and Endocrine Disorders. Eur J Clin Investig.. 2018;48((6)):e12927.
- [CrossRef] [PubMed] [Google Scholar]
- SUMOylation Controls CLOCK-BMAL1-Mediated Clock Resetting Via CBP Recruitment in Nuclear Transcriptional Foci. Biochim Biophys Acta.. 2015;1853((10 Pt A)):2697-708.
- [CrossRef] [PubMed] [Google Scholar]
- Transcriptional Architecture and Chromatin Landscape of the Core Circadian Clock in Mammals. Science.. 2012;338((6105)):349-54.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rhythmic CLOCK-BMAL1 Binding to Multiple E-box Motifs Drives Circadian Dbp Transcription and Chromatin Transitions. Nat Genet.. 2006;38((3)):369-74.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy-Induced Adaptations of the Central Circadian Clock and Maternal Glucocorticoids. J Endocrinol.. 2016;228((3)):135-47.
- [CrossRef] [PubMed] [Google Scholar]
- The Maternal-Placental-Fetal Interface: Adaptations of the HPA Axis and Immune Mediators Following Maternal Stress and Prenatal Alcohol Exposure. Exp Neurol.. 2022;355:114121.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Interactions of the Circadian CLOCK system and the HPA axis. Trends Endocrinol Metab.. 2010;21((5)):277-86.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Light, Timing of Biological Rhythms, and Chronodisruption in Man. Naturwissenschaften.. 2003;90((11)):485-94.
- [CrossRef] [PubMed] [Google Scholar]
- Chronodisruption: A Poorly Recognized Feature of CKD. Toxins (Basel).. 2020;12((3)):151.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The Circadian Timing System and Environmental Circadian Disruption: From Follicles to Fertility. Endocrinology.. 2016;157((9)):3366-73.
- [CrossRef] [PubMed] [Google Scholar]
- Light and Circadian Signaling Pathway in Pregnancy: Programming of Adult Health and Disease. Int J Mol Sci.. 2020;21((6)):2232.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Nightshift Work and Fracture Risk: The Nurses’ Health Study. Osteoporos Int.. 2009;20((4)):537-42.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effects of Shiftwork on Sleep and Menstrual Function in Nurses. Health Care Women Int.. 2002;23((6–7)):703-14.
- [CrossRef] [PubMed] [Google Scholar]
- Association Between Light at Night, Melatonin Secretion, Sleep Seprivation, and The Internal Clock: Health Impacts and Mechanisms of Circadian Disruption. Life Sci.. 2017;173:94-106.
- [CrossRef] [PubMed] [Google Scholar]
- Preterm Infants Born at Less Than 31 Weeks’ Gestation Have Improved Growth in Cycled Light Compared with Continuous Near Darkness. J Pediatr.. 2002;140((2)):192-9.
- [CrossRef] [PubMed] [Google Scholar]
- Reduced Lighting Does Not Improve Medical Outcomes in Very Low Birth Weight Infants. J Pediatr.. 2001;139((4)):527-31.
- [CrossRef] [PubMed] [Google Scholar]
- Environmental Light and the Preterm Infant. Semin Perinatol.. 2000;24((4)):291-8.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of light in the NICU on the Development of Circadian Rhythms in Preterm Infants. Semin Perinatol.. 2000;24((4)):247-57.
- [CrossRef] [PubMed] [Google Scholar]
- The Effects of Cycled Versus Noncycled Lighting on Growth and Development in Preterm Infants. Infant Behav Dev.. 1995;18((1)):87-95.
- [Google Scholar]
- Effect of Night and Day on Preterm Infants in a Newborn Nursery: Randomised Trial. Br Med J (Clin Res Ed).. 1986;293((6557)):1265-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Continuous Artificial Light Potentially Disrupts Central and Peripheral Reproductive Clocks Leading to Altered Uterine Physiology and Reduced Pregnancy Success in Albino Mice. Photochem Photobiol Sci. 2022;21:1217-1232.
- [CrossRef] [PubMed] [Google Scholar]
- Shift Work and Circadian Dysregulation of Reproduction. Front Endocrinol (Lausanne).. 2013;4:92.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]








