Translate this page into:
A Primer on Clinical Classification and Pathophysiology of Endometriosis

* Corresponding author: Dr. Deepak Modi, Molecular and Cellular Biology Laboratory, National Institute for Research in Reproductive and Child Health, Parel, Mumbai, India deepaknmodi@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Mishra A, Modi D. A Primer on Clinical Classification and Pathophysiology of Endometriosis. Fertil Sci Res. 2024;11:7. doi: 10.25259/FSR_7_2024
Abstract
Endometriosis is defined as the presence of endometrial tissue outside the uterus at an ectopic site. It affects around 18% of reproductive-age females worldwide. Currently, endometriosis is diagnosed laparoscopically and is classified into four different types, viz (1) Revised American Society for Reproductive Medicine (rASRM), (2) ENZIAN classification, (3) Endometriosis Fertility Index (EFI) and (4) American Association of Gynaecological Laparoscopists (AAGL). This grouping is proposed to categorise endometriosis on grounds of severity and progression. However, there is no optimal classification scheme and each of the systems has its own merits and shortcomings. Also, the therapeutic value of such a classification system is not yet clear. In terms of pathophysiology, Sampson’s retrograde menstruation theory is the most frequently recognised explanation for the origin of endometriosis, but factors like (1) genetic predisposition, (2) Oestrogen dependence, (3) progesterone resistance and (4) inflammation are thought to be involved in disease development and progression. However, our understanding of endometriosis is far from clear, and there is still much to learn and do. There is a need for an ideal categorisation strategy that appropriately reflects the severity of symptoms, disease progression and response to treatment. Till then, women with endometriosis will continue to suffer, and clinicians will remain in dilemma while managing this complex condition.
Keywords
Endometriosis
endometrium
oestrogen
progesterone
inflammation
Introduction
Endometriosis is the presence of endometrial epithelial and stromal cells at an ectopic location.[1] Dysmenorrhea, dyspareunia, dyschezia, dysuria, intermenstrual bleeding and chronic abdominopelvic pain, as well as subfertility, are signs of endometriosis, while asymptomatic cases can also occur. Endometriosis affects 5–10% of women of reproductive age, whereas 50–80% of women with pelvic pain and up to 50% of women with infertility have endometriosis.[2] Non-invasive methods cannot diagnose endometriosis. Based on the clinical history and therapeutic response, imaging techniques are recommended to diagnose the disease. Endometriotic lesions are detected using laparoscopy; more recently, transvaginal ultrasonography and magnetic resonance imaging (MRI) are considered alternates.[3,4] Treatment of patients with endometriosis may include medical therapy, surgical therapy or both. Medical treatment includes hormonal suppression and the reduction or elimination of menses to reduce pain, while surgical treatment targets relieving symptoms through ablative techniques or excision of lesions while still conserving reproductive function.[5] Endometriosis is a mysterious disease that is the subject of many theories. Sampson’s retrograde menstruation theory is the most frequently recognised explanation for the origin of endometriosis, while there are other views as well. However, other elements like genetic predisposition, oestrogen dominance, progesterone resistance and inflammation are also crucial for the establishment and development of endometriotic tissues in an ectopic location [Figure 1]. In this narrative review, we give an insight into the diagnostic classification strategies used in endometriosis and then focus on the current understanding of the pathophysiology of endometriosis.

- Graphical abstract. rASRM: Revised American Society for Reproductive Medicine, EFI: Endometriosis Fertility Index, AAGL:American Association of Gynaecological Laparoscopists.
Endometriosis
The word endometriosis is derived from the Greek words endon, meaning ‘within’, metra, meaning ‘uterus’ and osis, meaning ‘abnormal’ or diseased condition. It is a hormone-dependent gynaecological condition that is both complex and common where the functional endometrial glands and stroma, which are normally part of the innermost lining of the uterine cavity (the endometrium), are present outside the uterine cavity like ovaries, fallopian tubes, pelvic peritoneum, gastrointestinal tract, bladder, rectovaginal septum and less commonly, the pericardium and pleura.[6,7] However, with the advances in disease knowledge, this definition of endometriosis has been changed, and according to the new definition, ‘Endometriosis is a fibrotic condition where endometrial stromal and epithelia can be identified outside the uterus’.[1] About 6–10% of women globally have endometriosis, and the prevalence of endometriosis in developing countries is higher than in developed countries.[8]
Symptoms of Endometriosis
As shown in Figure 2, endometriosis manifests differently in different women. Most endometriosis patients are asymptomatic, and about 6–10% experience pelvic pain, intermenstrual bleeding, painful periods (dysmenorrhea), painful sex (dyspareunia), painful defecation (dyschezia), painful urination (dysuria) and infertility.[8–10].
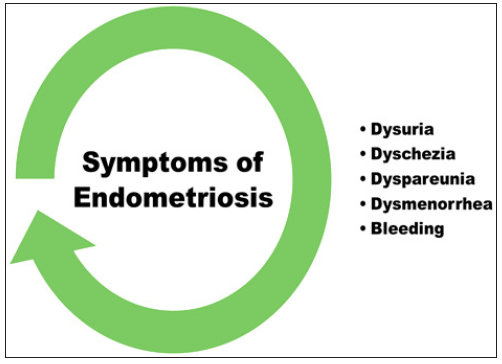
- Symptoms of endometriosis.
Classification of Endometriosis
A worrying problem with endometriosis treatment is the absence of a gold standard classification method. The efficacy of the existing classification methods is debatable, and there is currently no ideal classification scheme. Endometriosis has a wide range of clinical symptoms, and it is unclear what the correlation between disease severity and symptom severity is. To better categorise endometriosis, numerous efforts have been made. There are four standard classification systems for endometriosis[11]:
-
1.
Revised American Society for Reproductive Medicine (rASRM)
-
2.
ENZIAN classification
-
3.
Endometriosis Fertility Index (EFI)
-
4.
American Association of Gynaecological Laparoscopists (AAGL)
Revised American Society for Reproductive Medicine (rASRM)
The American Fertility Society (AFS) proposed the AFS score, a revolutionary technique, in 1979. The endometriosis stage was determined by a cumulative score based on the size of endometriotic lesions in the ovaries, peritoneum and fallopian tubes, as well as the level of adhesion at each of the sites mentioned above. The system was divided into four levels: I (mild to 5 points), II (moderate to 15 points), III (severe to 30 points), and IV (31 to 54 points, extensive).
However, there was no association between the illness stage and the clinical symptoms of pain and infertility in this classification method. As a result, this framework was modified in 1985, and endometriosis was divided into four stages: minimum, mild, moderate, and severe [Figure 3]. The score was divided into four categories: 1–5, 6–15, 16–40 and more than 40. Superficial small, isolated lesions less than 3 cm are classified as minimal, deep infiltrating large lesions greater than 3 cm without adhesions are classified as mild, deep infiltrating lesions greater than 3 cm with filmy adhesions are moderate and deep infiltrating lesions greater than 3 cm with dense adhesions are classified as severe.
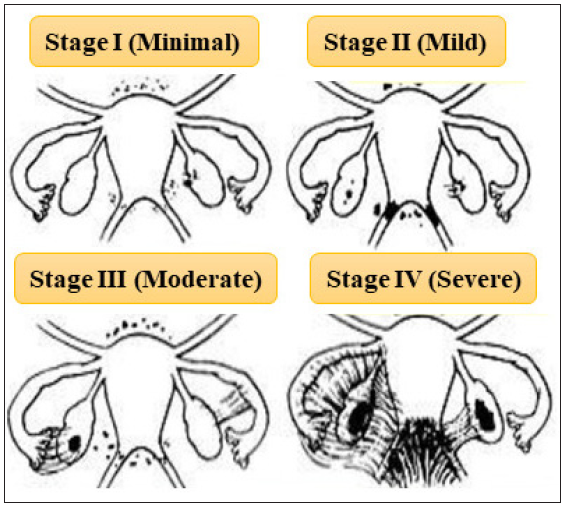
- rASRM classification for endometriosis (This figure is modified from the https://www.endometriosis-india.com/classification-of-endometriosis/)
The rASRM classification has the advantage of being widely used in recent years and being recognised on a global scale. It is very simple to use and useful for professionals when describing the severity of endometriosis to patients.
ENZIAN classification
The commonly used rASRM has some limitations because of its incomplete description of deep endometriosis. In terms of deep infiltrating endometriosis, the ENZIAN classification was designed to complement the rASRM classification rather than to replace it. When this categorisation was used, however, there was an unexpected partial overlap with the rASRM score. Two updates were made in 2010 and 2011 to eliminate the overlay amongst the rASRM classification systems and to make the ENZIAN classification system more user-friendly, as shown in Figure 4.
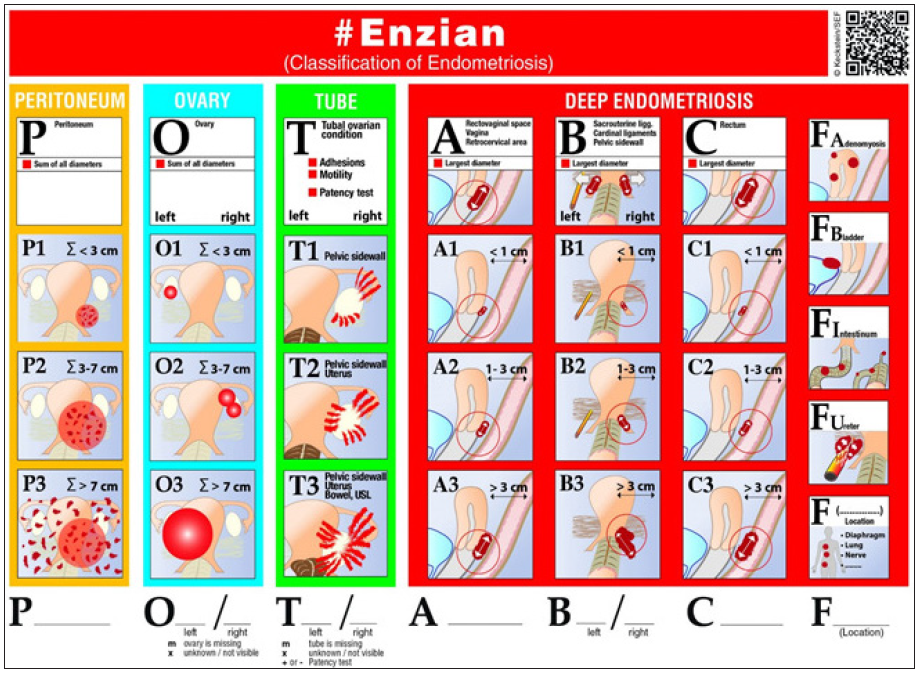
- The ENZIAN stage system for endometriosis in women. Overview of the ENZIAN categorisation, including compartments and organs that may be impacted (doi: 10.1111/aogs.14099).
Retroperitoneal structures were categorised into three compartments in the updated classification. The rectovaginal septum and vagina in the posterior region of the uterus comprise compartment A, the uterosacral ligament and pelvic walls comprise compartment B, and the sigmoid colon and rectum comprise compartment C. The invasiveness of the lesion is limited to 1 cm for grade I, 1–3 cm for grade II and more than 3 cm for grade III. The prefix ‘E’ indicates the presence of an endometriosis tumor. The afflicted compartment is designated by the lowercase English letter following the prefix, and the extent of the lesion is indicated by the number following the prefix. Bilateral disease is denoted by two lowercase English letters. Endometriosis can infiltrate local and distant organs in the following ways: ‘FA’ indicates adenomyosis, ‘FB’ for bladder involvement, ‘FU’ for intrinsic ureter involvement, ‘FO’ for other sites and ‘FI’ for intestine involvement.
The ENZIAN classification has the advantage of providing thorough descriptions of the retroperitoneal structures. The compartment can be subdivided into three sections, and each section’s severity can be described, as well as the severity of any remote lesions such as diaphragmatic and ureteral invasions. In addition, the ENZIAN classification can be employed using imaging modalities that are routinely employed for surgical planning. Third, the existence and intensity of various symptoms are related to and correlated with disease localisation and extent. However, there are several drawbacks. First, there is a low level of global acceptance for the ENZIAN categorisation. Second, patients could have trouble interpreting the ENZIAN classification due to the stage’s intricacy and lack of understanding of pelvic anatomy. Third, insufficient surgical dissection of the deep invasive lesions or performing an imaging study just without surgery will result in an incorrect ENZIAN score. Fourth, there are not enough studies to identify whether the ENZIAN categorisation is useful, even if imaging modalities predict it.
Endometriosis Fertility Index (EFI)
While the rASRM and ENZIAN provide an accurate classification of endometriosis, as these reflect disease progression over severity, the classification is inadequate to predict fertility in women with endometriosis. This led to the development of the EFI approach, as shown in Figure 5. This classification offers a definite advantage in terms of pregnancy outcome prediction in women with endometriosis.

- Endometriosis Fertility Index (EFI) system where the number shows the probability that a woman will become pregnant naturally after having endometriosis surgically confirmed (modified from doi: 10.12701/yujm.2020.00444 with permission of Elsevier)
The EFI approach takes into consideration past events like age, length of infertility and previous pregnancies. The function score is considered by assessing the function of the ovary, fallopian tube and fimbria on each side. The surgeon awards functional scores ranging from 0 to 4 using the following scale: absent or non-functional is assigned a score of 0; severe dysfunction is awarded a score of 1; moderate dysfunction is assigned a score of 2; mild dysfunction is assigned a score of 3, and normal is assigned a score of 4. Along with the least functional score, other surgical factors, such as the rASRM total score and endometriosis lesion score of rASRM, are considered. The EFI score is calculated, which can range from 0 to 10 points, and is determined by summing the historical and surgical values. The higher the EFI score, the greater the probability of fertility. The EFI score effectively represents the pregnancy rate better than the rASRM classification. The EFI score, however, is more difficult to use than the rASRM classification and ENZIAN score since it involves calculating and aggregating the scores of numerous categories.
American Association of Gynaecological Laparoscopists (AAGL)
Compared to the ASRM staging approach, the AAGL 2021 Endometriosis Classification enables the identification of objective intraoperative findings that accurately distinguish surgical complexity levels. The ASRM stage and the AAGL severity stage connect with pain and infertility symptoms equally. This new endometriosis classification was started in 2007 by the AAGL. Thirty endometriosis specialists were asked to rate the importance of the involvement site of the lesion on the outcomes of pain, infertility and surgical difficulty on a scale of 0 to 10. This system included all of the fundamental data deemed necessary for assessing a patient’s disease severity. Furthermore, the classification entails determining the surgical difficulty levels at four levels. Level 1 is the removal of superficial implants and simple thin avascular adhesions; level 2 is the removal of ovarian endometriomas, appendectomy and dense adhesions that do not involve the intestine and ureter; and level 3 is the removal of deep endometriosis that does not involve the vagina, bladder, bowel or ureter. Dense bowel and ureter adhesions, suture-required bladder surgery, ureterolysis and bowel surgery without resection (shaving) are all classified as level 3. Finally, level 4 is assigned when bowel resection with end-to-end anastomosis, ureteral reimplantation or anastomosis is required. Before surgery, the patient’s visual analogue scale scores and infertility histories are recorded to validate the scoring system. Even though it has been more than ten years since the classification was first developed, it has yet to be thoroughly validated and published.
In conclusion, ASRM, ENZIAN and EFI classifications are based on surgical assessment of the disease and its progression, and they meet the fundamental requirements of a clinical classification. The primary drawback of these classifications is their low diagnostic and prognostic use [Supplementary Table 1]. The only exception is the EFI classification, which aids in predicting fertility outcomes.[12] Recently, it has been proposed that rather than the conventional classification systems, transvaginal ultrasound (TVUS) and MRI-based diagnosis and staging may be better in surgical planning and infertility management in women with endometriosis.[13,14] However, the data available is insufficient for immediate clinical applications, and more studies from different parts of the world are needed to determine if the imaging-based diagnostic outcomes have any prognostic significance. The need is to create an approach where the classifications/staging systems, symptom assessment and diagnostic imaging are integrated to aid clinicians and patients in predicting the prognosis and determining appropriate treatments. Presently, for classifying this difficult and complex condition, there is still much to learn and to do. To appropriately reflect the severity of symptoms and diseases as well as to choose appropriate treatment options, an ideal categorisation should be devised, but this appears to be far from reality. Till then, clinicians will need to rely on multiple approaches to classify the disease based on the outcomes that are desired.
The Magnitude of the Problem
In general, endometriosis prevalence is 18%, and stage-specific prevalence ranges from 2% for stage IV to 20% for stage I. Endometriosis prevalence rates are 31%, 42% and 23%, respectively, among infertile women, those with chronic pelvic discomfort or asymptomatic women.[8]
Treatment
Current treatment for endometriosis is based on surgical/medical therapies or surgical followed by medical therapies. The surgical method includes laparoscopy, which is still the gold standard method for the diagnosis and removal of endometriosis.[15,16] The purpose of the laparoscopic approach is to destroy or remove all visually evident endometriotic tissues and heal the damage to organs caused by endometriosis, which restores normal anatomy.[15,17]
Medical therapy for endometriosis is based on the fact that sex steroid hormones modulate the ectopic endometriotic tissue and undergo cyclic changes the same as eutopic endometrium. The main targets are to reduce oestrogen levels (systemically and locally) and to restore pathogenesis-related (PR) resistance. The drugs targeting oestrogen secretion and oestrogen receptor (ER) activity are GnRH-analogues, GnRH antagonists, aromatase inhibitors and selective oestrogen receptor modulators (SERMs). Drugs targeting progesterone or PR activity in the pathogenesis of endometriosis are progestin (oral, intravaginal, intrauterine and subcutaneous) and selective progesterone receptor (PR) modulators.[3,18] However, available therapies, either surgical or medical, are not appropriate for the long term due to multiple side effects and a high recurrence rate of disease. Therefore, an ideal treatment for endometriosis should be long-standing, have limited side effects, be less painful, balance hormone and receptor levels and improve fertility.[18]
Aetiology of Endometriosis
The aetiology of endometriosis is complex and is based on multiple factors. Various theories have been put forth to explain the mechanisms that may cause endometriosis. These include the theory of retrograde menstruation, metaplasia, hormone disbalance, oxidative stress and inflammation, immune dysfunction, apoptosis suppression, alteration of endometrial cell fate, genetics and stem cell dysfunction [Figure 6].
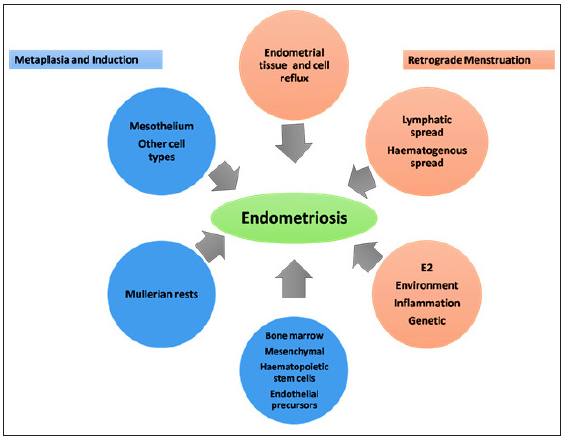
- Theories regarding the pathophysiology of endometriosis (modified from https://doi.org/10.1016/j.fertnstert.2012.06.029).
Amongst these, Sampson’s theory of retrograde menstruation[19,20] is the most widely accepted theory of endometriosis. It is suggested that the normal endometrium undergoes reverse flow of menstrual fluid containing endometrial debris via the fallopian tubes into the peritoneum, and the tissue fragments implant and grow at ectopic locations, leading to endometriosis.
However, retrograde menstruation occurs in 70–90% of women, but only 10% of women have endometriosis.[19,21,22] Also, endometriosis is observed in organs distant from the peritoneum like the brain, diaphragm, pleura and pericardium.[23–25] Further, the prevalence of endometriosis is not higher in all women with a retroverted uterus, endometrial hyperplasia, leiomyomata and menstrual blood in the peritoneal cavity.[19,20] Therefore, retrograde menstruation theory explains only the physical displacement of endometrial tissues; other factors are required for the development of endometriotic implants. Suppose endometriosis is to form from the retrograde passage of endometrial tissues. In that case, immune clearance must be avoided, there must be an attachment to the peritoneal epithelium, invasion of the epithelium, creation of local neovascularity and ongoing growth and survival of the endometrial implants.[26] Several well-supported molecular characteristics of endometriosis have been discovered through investigations into the pathophysiology of this condition,[27] including:
-
1.
Genetic predisposition
-
2.
Oestrogen dependence
-
3.
Progesterone resistance
-
4.
Inflammation
Genetic predisposition
It is believed that endometriosis has genetic influences that contribute to its pathophysiology. Familial studies, linkage analyses, genetic association studies and genome-wide association studies (GWAS) have provided insight into the pathophysiology of endometriosis via correlating cellular processes to the development of the disease. However, many parts of the disease’s aetiology are still unknown. First-degree relatives of women with severe endometriosis are at six times more risk than relatives of women who are unaffected. Studies on monozygotic twins show significant concordance rates for endometriosis with histological confirmation.[28] A linkage analysis study covered almost 1100 families and demonstrated that endometriosis is associated with loci on chromosomes 7p13-15 and 10q26.[29,30] Genetic association studies demonstrated that polymorphism in DNA repair pathway genes (XRCC1, hMLH1), proliferation and differentiation-associated genes (homeobox genes, p27, PLA2G2A, LAMA, KAZN), apoptotic genes (FAS, calpains, KRAS proto-oncogene, SIRT1 and BCL6), tumour suppressor genes (TP53, BCL6, SIRT1), Detoxification genes (CYP genes, GST genes), inflammatory and autoimmunity genes (IL-16, IL-1β, TNF-α, ICAM-1, COX-2, BsrBI, FCRL3), angiogenesis-related genes (FGFR2, VEGF, AKT1, TYMP and other genes), hormonal genes (LH, FSH, ERα) and genes in diverse pathway genes are associated with the risk of developing endometriosis in a different population.[28]
In a meta-analysis based on GWAS performed in a Japanese female population, four prevalent SNPs were observed near and within the IL1A region, indicating it is a candidate gene.[31] Four SNPs (rs227849, rs4703908, rs2479037 and rs966674) were also found to be strongly linked with endometrioma risk in a different GWAS that included 2019 diagnosed patients.[32] The genetic variant rs12700667 on 7p15.2 was found in populations of European and Japanese descent, and a correlation between rs7521902 at 1p36.12 close to WNT4 was confirmed in another GWAS with 4604 patients.[33] Moreover, another risk locus at 4q12 (rs17773813) was discovered in a GWAS involving 1840 patients of Icelandic descent.[34]
While the GWAS studies are immensely useful in understanding the genetics of endometriosis, very few of the candidate genes have been experimentally validated to show a functional effect. Thus, these results are considered, at best, associative to explain the genetic predisposition theory of endometriosis.
Oestrogen dependence
Steroid hormones are crucial for preserving endometrial physiology and are thought to be involved in the aetiology of endometriosis. Oestradiol, an oestrogen steroid hormone, is necessary for the development of endometriotic implants.[35] In addition to oestradiol being produced in the ovary, endometriotic lesions also produce oestradiol locally.[36,37] The production of oestradiol promotes the synthesis of prostaglandins, which, in turn, drives oestrogen synthesis locally, resulting in a feed-forward system.[27,38,39]
Oestrogen affects the target tissue by acting on its receptors, namely the ERs, which include the oestrogen receptor alpha (ERα) and oestrogen receptor beta (ERβ). In the normal endometrium, ERα is hormonally controlled and necessary for the proliferation of endometrial cells; ERβ has anti-proliferative and inflammatory functions.[40–43] Ectopic endometrial tissues overexpress ERβ, which causes ERα to be suppressed and reduces ERα-mediated activation of the PR. This altered ERα/ERβ favours cell survival, maintains inflammation, and may contribute to progesterone resistance.[2]
Progesterone resistance
In its natural state, progesterone causes the endometrium to decidualise, inhibits oestrogen-dependent endometrial growth and serves as an anti-inflammatory.[44,45] Progesterone resistance in endometriosis was hypothesised as a result of in vitro experiments that revealed progesterone was unable to trigger the formation of retinoic acid in endometriosis lesions.[46,47] Since retinoic acid is not produced, endometriotic lesions have higher than normal levels of oestradiol, which promotes further growth.[48,49] In addition, endometriosis has a low PR isoform B (PR-B) to PR isoform A (PR-A) ratio. PR-B is a powerful transactivator in response to progesterone, and PR-A is a dominant repressor,[50] but it is possible that reduced PR-B is a contributing factor in progesterone resistance.[46,51–53].
Beyond the expression of the receptors, endometrial gene expression analysis has revealed that endometriosis-affected women have reduced expression of progesterone target genes during the window of embryo implantation.[54,55] These genes play a role in immunomodulation and decidualisation, indicating that the eutopic endometrium of women with endometriosis is also progesterone-resistant.[51,53] In the normal endometrium, HOXA10 is a direct target of PR in endometrial stromal cells and drives the expression of many progesterone-regulated genes.[56,57] The expression of HOXA10 is also reduced in the eutopic and ectopic endometrium of women with endometriosis. Epigenetic modifications also have a role in progesterone resistance.[58,59] Studies have demonstrated that the promoter of the PRB gene, HOXA10, DNMTs (DNA methyltransferases) and steroidogenic factor-1 (SF-1) are hypermethylated in eutopic endometrium.[46,60] Furthermore, endometriosis tissues have hypomethylation of ERβ, which may be a reason for the increased expression of ERβ.[46,61]
At present, it appears that the acquisition of oestrogen dominance and progesterone resistance could be a possible reason for the development of endometriotic lesions at ectopic locations. Indeed, we and others have shown experimentally that oestrogen is necessary for the development of endometriotic lesions in mouse models[62,63]; mice lacking ER isoforms do not effectively develop endometriosis.[64,65]
Inflammation
A key characteristic of endometriosis is inflammation; however, it is unknown whether this condition contributes to the onset of the disease or is responsible for its progression. The interaction between immune and hormonal systems significantly influences the pathogenesis and development of endometriosis. E2 has a notable function in the promotion of inflammation by inducing the release of cytokines and prostaglandins from peritoneal macrophages[66] by the action of ERβ.[67] ERα has a dual role, with both anti- and proinflammatory actions.[63] However, there is an imbalance in the sex steroid hormones’ actions in endometriosis, with oestrogens playing a significant part in the condition’s exaggerated proinflammatory state.
In endometriosis, cytokine concentrations are abnormally elevated. Multiple studies have revealed elevated levels of TNF-α, IL-1β, IL-6, IL-8, CCL2, CCL5 and VEGF in affected patients.[68–73] The nuclear factor kappa-light-chain-enhancer of activated B cells is activated by this influx of proinflammatory cytokines, which intensifies the inflammatory response and counteracts the benefits of progesterone.[74] It has also been noted that immune cell distribution is aberrant. Peritoneal fluid lymphocyte numbers are higher even when the total number of lymphocytes in the blood is unchanged.[75,76] Additionally, there is an increase in peritoneal macrophage concentrations, which paradoxically drive endometriotic lesions by promoting angiogenesis.[77–80]. The reduced cytotoxicity of natural killer cells may also improve lesion survival.[80–83] Furthermore, it is observed that the sera of the diseased women include antibodies to ovarian and endometrial antigens.[84–86] These findings imply that endometriosis is associated with autoimmune disorders. This association is further supported by a meta-analysis showing a statistically significant correlation between endometriosis and at least one classic autoimmune disease, including Systemic Lupus Erythematosus (SLE), Sjögren’s Syndrome (SS), Rheumatoid Arthritis (RA), Autoimmune Thyroid Disorder, Coeliac Disease (CLD), Multiple Sclerosis (MS), and Inflammatory Bowel Disease (IBD).
Thus, it appears that endometriosis has a broad inflammatory environment that extends outside the pelvis and is marked by the presence of proinflammatory cytokines and changes in the populations of circulating immune cells. Intriguingly, an association of polymorphisms in inflammation-related genes is also reported.[87–89] Thus, genetic predisposition and the creation of an inflammatory milieu due to oestrogen dominance and progesterone resistance may contribute to endometriosis.
Conclusion
Clinically, endometriosis is classified as ASRM, ENZIAN or EFI systems that are based on the surgical assessment of the disease and its progression; the primary drawback of these classifications is their low diagnostic and prognostic use. There is a need for an appropriate diagnostic and classification system that should be minimally invasive, determine the disease severity, predict its prognosis and aid in choosing the best therapeutic modality. However, such an approach is unavailable and clinicians will need to diagnose the women by imaging and treat the women based on clinical symptomatology. To aid in developing an ideal classification system for endometriosis, we need to understand its pathophysiology. Current data suggests that genetic predisposition, oestrogen dominance, progesterone resistance and inflammation are crucial for lesion establishment and progression at ectopic locations. Integrating such laboratory findings and clinical data will help us develop better management of women with endometriosis.
Acknowledgements
The inputs from the members of the DM lab during review preparation are greatly acknowledged.
Authors’ contribution
Conception and design: AM, DM
Collection and assembly of data: AM
Manuscript writing: All authors
Final approval of manuscript: All authors
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient consent is not required as there are no patients in this study.
Financial support and sponsorship
The manuscript bears the NIRRCH ID: REV/1656/10-2023. The study was supported by grants from the Department of Science and Technology (SERB) (SR/SO/HS-0277/2012), Government of India to DM. AM is the recipient of the senior research fellowship from ICMR and the University Grants Commission (UGC), Government of India (# 2121330598). DM lab is funded by grants from ICMR, the Government of India.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Time to Redefine Endometriosis Including its Pro-Fibrotic Nature. Hum Reprod. 2018;33:347-52.
- [CrossRef] [PubMed] [Google Scholar]
- Endometriosis is a Chronic Systemic Disease: Clinical Challenges and Novel Innovations. Lancet. 2021;397:839-52.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Management of Endometriosis. Obstet Gynecol. 2018;131:557-71.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic Resonance Imaging for Deep Infiltrating Endometriosis: Current Concepts, Imaging Technique and Key Findings. Insights Imaging. 2021;12:105.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Endometriosis: An Update on Diagnosis and Medical Management. B C Med J. 2021;63:158-63.
- [Google Scholar]
- Introduction to Endometriosis. 2015;1–5
- [CrossRef] [PubMed]
- Beyond the Boundaries-Endometriosis: Typical and Atypical Locations. Curr Probl Diagn Radiol. 2011;40:219-32.
- [CrossRef] [PubMed] [Google Scholar]
- A Systematic Review on the Prevalence of Endometriosis in Women. Indian J Med Res. 2021;154:446-54.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr Obstet Gynecol Rep. 2017;6:34-41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Endometriosis and Infertility. J Assist Reprod Genet. 2010;27:441-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Classification of Endometriosis. Yeungnam Univ J Med. 2021;38:10-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Classification/Staging Systems for Endometriosis: The State of the Art. Gynecol Reprod Endocrinol Metab. 2020;1:14-22.
- [PubMed] [Google Scholar]
- Advances in Imaging for Assessing Pelvic Endometriosis. Diagnostics (Basel). 2022;12:2960.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Imaging Modalities for the Non-invasive Diagnosis of Endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Laparoscopic Surgery for Endometriosis. Cochrane Database Syst Rev. 2020;10:CD011031.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Surgical Treatment of Different Types of Endometriosis: Comparison of Major Society Guidelines and Preferred Clinical Algorithms. Best Pract Res Clin Obstet Gynaecol. 2018;51:102-10.
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic Surgery for Endometriosis. Cochrane Database Syst Rev. 2014;10:CD011031.
- [CrossRef] [PubMed] [Google Scholar]
- Hormonal Therapy for Endometriosis: From Molecular Research to Bedside. Eur J Obstet Gynecol Reprod Biol. 2017;209:61-6.
- [CrossRef] [PubMed] [Google Scholar]
- Theories on the Pathogenesis of Endometriosis. Int J Reprod Med. 2014;2014:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Session 1: Pathogenesis and Progression of Endometriosis 1 Implantation Versus Infiltration: The Sampson Versus the Endometriotic Disease Theory. Gynecol Obstet Inves. 1999;47:3-10.
- [CrossRef] [PubMed] [Google Scholar]
- Endometriosis Still a Challenge. J Med Life. 2014;7:349-57.
- [PubMed] [PubMed Central] [Google Scholar]
- Stem Cells and the Pathogenesis of Endometriosis. Ann N Y Acad Sci. 2008;1127:106-15.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Extrapelvic Endometriosis: A Rare Entity or an Under Diagnosed Condition? Diagn Pathol. 2013;8:1-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Micrometastasis of Endometriosis to Distant Organs in a Murine Model. Oncotarget. 2019;10:2282-91.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pericardial, Pleural and Diaphragmatic Endometriosis in Association with Pelvic Peritoneal and Bowel Endometriosis: A Case Report and Review of the Literature. Wideochirurgia I Inne Tech Maloinwazyjne. 2012;7:122-31.
- [CrossRef] [PubMed] [Google Scholar]
- Pathogenesis and Pathophysiology of Endometriosis. Fertil Steril. 2012;98:511-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Defining the Genetic Profile of Endometriosis (Review) Exp Ther Med. 2019;3267–81
- [CrossRef] [PubMed] [Google Scholar]
- Significant Evidence of One or more Susceptibility Loci for Endometriosis with Near-Mendelian Inheritance on Chromosome 7p13-15. Hum Reprod. 2007;22:717-28.
- [CrossRef] [PubMed] [Google Scholar]
- High-density Fine-mapping of a Chromosome 10q26 Linkage Peak Suggests Association between Endometriosis and Variants Close to CYP2C19. Fertil Steril. 2011;95:2236-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Meta-analysis of Genome-wide Association Scans for Genetic Susceptibility to Endometriosis in Japanese Population. J Hum Genet. 2010;55:816-21.
- [CrossRef] [PubMed] [Google Scholar]
- Genome-Wide Association Study Link Novel Loci to Endometriosis. PLoS One. 2013;8:e58257.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Genome-wide Association Meta-analysis Identifies New Endometriosis Risk Loci. Nat Genet. 2012;44:1355-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Common Variants Upstream of KDR Encoding VEGFR2 and in TTC39B Associate with Endometriosis. Nat Commun. 2016;7:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Endometriosis. N Engl J Med. 2010;362:2389-98.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Aromatase in Endometriosis and Uterine Leiomyomata. J Steroid Biochem Mol Biol. 2005;95:57-62.
- [CrossRef] [PubMed] [Google Scholar]
- Anastrazole and Oral Contraceptives: A Novel Treatment for Endometriosis. Fertil Steril. 2005;84:300-4.
- [CrossRef] [PubMed] [Google Scholar]
- Endometriosis and Nuclear Receptors. Hum Reprod Update. 2019;25:473-85.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- ERβ- and Prostaglandin E2-regulated Pathways Integrate Cell Proliferation via Ras-like and Estrogen-regulated Growth Inhibitor in Endometriosis. Mol Endocrinol. 2014;28:1304-15.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Estrogen Receptors Alpha (ERα) and Beta (ERβ): Subtype-Selective Ligands and Clinical Potential. Steroids. 2014;90:13-29.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Reversal of fortune: Estrogen Receptor-β in Endometriosis. J Mol Endocrinol. 2016;57:F23-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Spatial and Temoporal Changes in the Expression of Steroid Hormone Receptors in Mouse Model of Endometriosis. J Assist Reprod Genet. 2020;37:1069-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mouse Model for Endometriosis is Characterized by Proliferation and Inflammation but not Epithelial-to-mesenchymal Transition and Fibrosis. J Biosci. 2020;45
- [PubMed] [Google Scholar]
- Global Gene Profiling in Human Endometrium during the Window of Implantation. Endocrinology. 2002;143:2119-38.
- [CrossRef] [PubMed] [Google Scholar]
- Progesterone Receptor Regulates Decidual Prolactin Expression in Differentiating Human Endometrial Stromal Cells. Endocrinology. 1999;140:4809-20.
- [CrossRef] [PubMed] [Google Scholar]
- Progesterone Resistance in Endometriosis: Origins, Consequences and Interventions. AOGS. 2017;96:623-32.
- [CrossRef] [PubMed] [Google Scholar]
- Altered Retinoid Uptake and Action Contributes to Cell Survival in Endometriosis. J Clin Endocrinol Metab. 2010;95:300-9.
- [CrossRef] [PubMed] [Google Scholar]
- Estrogen Biosynthesis in Endometriosis: Molecular Basis and Clinical Relevance. J Mol Endocrinol. 2000;25:35-42.
- [CrossRef] [PubMed] [Google Scholar]
- Progesterone Resistance in Endometriosis: Link to Failure to Metabolize Estradiol. Mol Cell Endocrinol. 2006;248:94-103.
- [CrossRef] [PubMed] [Google Scholar]
- The Opposing Transcriptional Activities of the Two Isoforms of the Human Progesterone Receptor are Due to Differential Cofactor Binding. Mol Cell Biol. 2000;20:3102-15.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Expression Profiling of Endometrium from Women with Endometriosis Reveals Candidate Genes for Disease. Based Implantation Failure and Infertility. Endocrinology. 2003;144:2870-81.
- [CrossRef] [PubMed] [Google Scholar]
- Estrogen Receptor-β, Estrogen Receptor-α, and Progesterone Resistance in Endometriosis. Semin Reprod Med. 2010;28:36-43.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gene Expression Analysis of Endometrium Reveals Progesterone Resistance and Candidate Susceptibility Genes in Women with Endometriosis. Endocrinology. 2007;148:3814-26.
- [CrossRef] [PubMed] [Google Scholar]
- Loss of HOXA10 Causes Endometrial Hyperplasia Progressing to Endometrial Cancer. J Mol Endocrinol. 2022;69:431-44.
- [CrossRef] [PubMed] [Google Scholar]
- Dynamics of HOXA10 Expression in Ectopic Endometrium of a Mouse Model of Endometriosis. Fertil Sci Res. 2023;10:195-204.
- [Google Scholar]
- Progesterone-regulated Endometrial Factors Controlling Implantation Arpita. Am J Reprod Immunol. 2016;75:237-45.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The Role of Hox Genes in Female Reproductive Tract Development, Adult Function, and Fertility. Cold Spring Harb Perspect Med. 2016;6:a023002.
- [Google Scholar]
- Molecular Biology of Endometriosis: From Aromatase to Genomic Abnormalities. Semin Reprod Med. 2015;33:220-4.
- [CrossRef] [PubMed] [Google Scholar]
- Progesterone Resistance in Endometriosis: Current Evidence and Putative Mechanisms. Int J Mol Sci. 2023;24:6992.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Promoter Hypermethylation of Progesterone Receptor Isoform B (PR-B) in Endometriosis. Epigenetics. 2006;1:106-11.
- [CrossRef] [PubMed] [Google Scholar]
- Estrogen is Essential but not Sufficient to Induce Endometriosis. J Biosci. 2017;42:251-63.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Estrogen Receptor Signaling Required for Endometriosis-like Lesion Establishment in a Mouse Model. Endocrinology. 2012;153:3960-71.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Estrogen Receptor B Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis Article Estrogen Receptor B Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell. 2015;163:960-74.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Estrogen Receptors and Endometriosis. Int J Mol Sci. 2020;21:1-17.
- [CrossRef] [PubMed] [Google Scholar]
- 17Β-estradiol and Lipopolysaccharide Additively Promote Pelvic Inflammation and Growth of Endometriosis. Reprod Sci. 2015;22:585-94.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Role of Estrogen Receptor-β in Endometriosis. Bone. 2012;30:39-45.
- [CrossRef] [PubMed] [Google Scholar]
- Peritoneal Fluid Concentrations of the Cytokine RANTES Correlate with the Severity of Endometriosis. Am J Obstet col. 1993;169:1545-9.
- [CrossRef] [PubMed] [Google Scholar]
- Monocyte Chemotactic Protein-1 Concentration in Peritoneal Fluid of Women with Endometriosis and its Modulation of Expression in Mesothelial Cells. Fertil Steril. 1997;67:1065-72.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin-8 induces Proliferation of Endometrial Stromal Cells: A Potential Autocrine Growth Factor. J Clin Endocrinol Metab. 1998;83:1201-5.
- [CrossRef] [PubMed] [Google Scholar]
- Chemotactic Protein-1 Expression by Ectopic Endometrial Cells of Women with Endometriosis. J Clin Endocrinol Metab. 2000;85:896-904.
- [CrossRef] [PubMed] [Google Scholar]
- Peritoneal Fluid Lnterleukin‐1β and Tumor Necrosis Factor in Patients with Benign Gynecologic Disease. Am J Reprod Immunol. 1991;26:62-7.
- [CrossRef] [PubMed] [Google Scholar]
- Serum Cytokines as Biomarkers for Nonsurgical Prediction of Endometriosis. Eur J Obstet Gynecol Reprod Biol. 2008;137:240-6.
- [CrossRef] [PubMed] [Google Scholar]
- Involvement of the Nuclear Factor-κB Pathway in the Pathogenesis of Endometriosis. Fertil Steril. 2010;94:1985-94.
- [CrossRef] [PubMed] [Google Scholar]
- Cytotoxic T-cells in Peripheral Blood in Women with Endometriosis. Geburtshilfe Frauenheilkd. 2013;73:1042-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Immunologic Aspects of Human Endometriosis. Am J Reprod Immunol. 1984;6:33-6.
- [CrossRef] [PubMed] [Google Scholar]
- Peritoneal Fluid Cell Populations in Infertility Patients. Fertil Steril. 1981;35:696-8.
- [CrossRef] [PubMed] [Google Scholar]
- Peritoneal Macrophages and Infertility: The Association between Cell Number and Pelvic Pathology. Fertil Steril. 1985;44:772-7.
- [CrossRef] [PubMed] [Google Scholar]
- Monocyte-mediated Enhancement of Endometrial Cell Proliferation in Women with Endometriosis. Fertil Steril. 1994;61:78-84.
- [CrossRef] [PubMed] [Google Scholar]
- Peritoneal Natural Killer Cytotoxicity and CD25+CD3+ Lymphocyte Subpopulation are Decreased in Women with Stage III-IV Endometriosis. Hum Reprod. 1995;10:2671-5.
- [CrossRef] [PubMed] [Google Scholar]
- Decreased Natural Killer Cell Activity in Endometriosis Patients: Relationship to Disease Pathogenesis. Fertil Steril. 1994;62:1086-8.
- [CrossRef] [PubMed] [Google Scholar]
- Platelets Impair Natural Killer Cell Reactivity and Function in Endometriosis through Multiple Mechanisms. Hum Reprod. 2017;32:794-810.
- [CrossRef] [PubMed] [Google Scholar]
- IL15 Promotes Growth and Invasion of Endometrial Stromal Cells and Inhibits Killing Activity of NK Cells in Endometriosis. Reproduction. 2016;152:151-60.
- [CrossRef] [PubMed] [Google Scholar]
- Endometriosis: The Prevalence of Endometrial Immunoglobulin g Antibodies in Patients with Endometriosis. Hum Reprod. 1995;10:1214-9.
- [CrossRef] [PubMed] [Google Scholar]
- Autoimmunity to Endometrium and Ovary in Endometriosis. Obstet Gynecol Surv. 1983;38:587-9.
- [PubMed] [Google Scholar]
- Identification and Validation of Novel Serum Markers for Early Diagnosis of Endometriosis. Hum Reprod. 2012;27:408-17.
- [CrossRef] [PubMed] [Google Scholar]
- Association between Polymorphisms of Cytokine Genes and Endometriosis: A Comprehensive Systematic Review and Meta-analysis Shulin. J Reprod Immunol. 2023;158:103969.
- [CrossRef] [PubMed] [Google Scholar]
- Genetics and Inflammation in Endometriosis: Improving Knowledge for Development of New Pharmacological Strategies. Int J Mol Sci. 2021;22:9033.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Genetic Variation at Chromosome 2q13 and its Potential Influence on Endometriosis Susceptibility through Effects on the IL-1 Family. Reprod Sci. 2018;25:1307-17. https://doi.org/10.1016/j.fertnstert.2012.06.029
- [CrossRef] [PubMed] [Google Scholar]








