Translate this page into:
A randomized controlled trial of combination of letrozole and clomiphene citrate or letrozole alone for induction of ovulation in women with clomiphene citrate-resistant polycystic ovary syndrome attending infertility centre
Address for correspondence: Uma Shrivastava, Infertility Centre, Bijuli Bazar, Kathmandu 12521, Nepal. E-mails: umasuminas@gmail.com, dr. ushrivastava@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
To measure the efficacy of combined clomiphene citrate (CC) and letrozole versus letrozole alone in the induction of ovulation in CC-resistant polycystic ovary syndrome (PCOS) cases.
Design:
Outcome assessor-blinded randomized controlled trial.
Setting:
Infertility Centre, Kathmandu, Nepal.
Participants:
One hundred and ninety-eight CC-resistant PCOS cases (99 in each group).
Intervention:
Treatment group received CC 100 mg once a day starting day 3 or 5 of menstrual cycle alternately for 3 days and letrozole 5 mg once a day starting day 4 or 6 of menstrual cycle alternately for 3 days. The comparison group received letrozole 5 mg once a day starting day 3 or 5 of menstrual cycle for 5 days.
Main outcome measures:
Number of mature follicles (18–22 mm), and increase in endometrial thickness.
Results:
Among total participants, 159 (81 in treatment and 78 in comparison group) were analyzed. Number of matured follicles was higher in the treatment group (1.59 ± 0.16) when compared with the comparison group (1.21 ± 0.56) which was statistically significant (P = 0.03) at 95% confidence interval. There was significant difference in the mean endometrial thickness (P = 0.01) in the treatment group (9.04 ± 0.15) when compared with the comparison group (8.51 ± 0.12). The conception rate was 35.80% in treatment group and 32.05% in the comparison group which was not significant in the Chi-squared test (P = 0.62).
Conclusion:
The combined CC and letrozole treatment is better than letrozole alone for ovulation induction in CC-resistant PCOS cases.
Trial registration:
Clinical Trials Registry-India CTRI/2019/01/017311
Keywords
Clomiphene citrate
infertility
letrozole
PCOS
pregnancy rate
INTRODUCTION
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorder in women of reproductive age group with prevalence of 12% to 18%.[1,2] It affects ovulatory function of a woman[3] and is the most prevalent cause of anovulatory infertility.[4] Apart from obesity in majority of PCOS women, frequent symptoms which they bear are oligoanovulation, clinical, or biochemical hyperandrogenism. The functional defects in PCOS can be observed by hormone assay, mainly follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, anti-Müllerian hormone (AMH), and androgen profile. The structural abnormalities in the ovaries and endometrium can be visualized by transvaginal ultrasound. In addition, there is the presence of numerous cortical follicles in both ovaries which bear a black pearl necklace shape or polycystic look.
Hence, to diagnose PCOS, Rotterdam criteria (2003) are still used as strong backbone which has been proved for good reproductive outcome. According to this criteria, PCOS can be diagnosed if two of the following three oligoanovulation, clinical or biochemical hyperandrogenism, and both ovaries with more than 12 follicles are observed.[5]
Ovulation induction is one of the major therapeutic procedures for women with PCOS to restoreovulation and fertility.[6] Clomiphene citrate (CC)-selective estrogen receptor modulator has been considered to be the first-line therapy for ovulation induction to women with PCOS with ovulation rate of 70% to 85% per cycle, pregnancy rate of 30% to 40%, and live birth rate of 50% to 60%.[7] However, it has some disadvantages that it is antiestrogenic on endometrium, there is greater chance of multiple pregnancies and may lead to hyperstimulation on its use. In addition, resistance to CC is common and occurs in about 15% to 40% of the cases of PCOS.[8] Resistance is defined as failure to ovulate with CC in six cycles.[9]
Letrozole, an aromatase inhibitor, is a newer drug equally effective as first-line treatment CC for ovulation induction in anovulation. It is beneficial due to absence of antiestrogenic effects. In addition, there is a least possibility of multiple pregnancy on its use. It is a good alternative for CC-resistant PCOS. Letrozole has been found to be better in ovulation and pregnancy rate in PCOS patient.[10]
In recent years, combined CC and letrozole along with metformin have been successfully used for the treatment of CC-resistant PCOS cases.[11] Some studies did not see any advantage to the use of letrozole over CC.[12] CC and letrozole have been used in combination with metformin for better outcome.[8] Few studies have shown a beneficial effect on endometrium, increment in the size of follicles, achievement of pregnancy with the use of combined letrozole and CC, and recommended for this therapy before administration of stronger drugs such as gonadotropins or laparoscopic ovarian drilling in CC-resistant PCOS women.[13,14,15]
The prevalence of PCOS-related infertility in Nepal is still unknown.[16] There are increasing cases of PCOS in infertility clinics. Majority of these cases have undergone CC induction without any conception, probably due to resistant to CC. Universally, it is still unclear which therapy is best option for the treatment of CC-resistant PCOS cases. None of the studies have been performed as combined therapy with alternated day administration of CC and letrozole. Very few studies have determined efficacy of combined CC and letrozole therapy. This study is trying to determine the potentiated effect of CC when letrozole was added alternate to CC in the same cycle so as to recover quality ovulation. In addition, the major goal of treatment is to recover ovulation as well as fertility through possible treatment options. In a preliminary study, positive results were observed with good ovulation and pregnancy outcome in some cases treated with this regimen at the infertility centre before planning for this study.
The objective of the study was to compare the efficacy of combined letrozole and CC with letrozole alone for ovulation induction among CC-resistant PCOS women attending infertility centre. The study also aimed to compare the rate of ovulation, pregnancy, and miscarriage in both the groups as outcome of the study.
No funding was received for this study. The budget required for the trial was borne by the infertility centre itself. The trial was registered in Clinical Trials Registry India. The registration number is CTRI/2019/01/017311. The study registration number in NHRC Nepal is 713/2018.
METHODOLOGY
We designed a quantitative interventional study to assess the induction of ovulation in both groups. A randomized controlled trial was conducted to measure the efficacy of combined CC and letrozole versus letrozole alone in the induction of ovulation in CC-resistant PCOS cases. This was an outcome assessor-blinded randomized controlled trial conducted among the CC-resistant PCOS women attending the Infertility Centre, Bijulibazar, Kathmandu, Nepal. This was a parallel trial where the participants were allocated to the treatment and comparison groups in 1:1 ratio. Lottery method was used to randomly assign participants into either of the group. Participants were asked to pick a folded sheet where random numbers were alloted and then they were assigned to the respective groups based on the selected number. The random numbers were generated using Microsoft Excel.
Women of age 20 to 40 years with the history of PCOS and resistant to CC were included in the study. Considering the lower success rate of CC due to antiestrogenic effect in women above 40 years of age[17] and because of the fact that the quality of follicles gets diminished after 40 years, women above the age of 40 years were excluded from the study. In addition, women attending infertility centre with the history of PCOS who were in first-line treatment and not yet reached six cycles of CC treatment, women with premature ovarian insufficiency, women whose husband has oligoazoospermia, and women with hypothyroidism and hyperprolactinemia were excluded from the study.
The study commenced from January 2019 and ended on June 2020. The recruitment of the participants started on February 2019 and ended on April 2020. Initially, it was planned to complete the study by December 2019 but data safety and monitoring board suggested to enroll more participants to cover the loss to follow-up participants and those who would later be excluded from analysis. Hence, with the permission from the ethical board, study was extended to June 2020. Given the coronavirus disease 2019 situation, the board decided to stop the study in June 2020 though few participants were left to follow-up. Follow-up of the participants was carried out in each cycle of their menstrual period.
The primary outcome of this study was to increase in number of mature follicles (18–22 mm), rise in serum estradiol level, and increase in endometrial thickness. Number of matured follicles and endometrial thickness were measured through transvaginal sonography (TVS) in every cycle of the treatment and recorded duly. Pregnancy and miscarriage were taken as the secondary outcome. For the secondary outcome, participants who became pregnant in the due course were followed up through ante-natal care until 3 months and their outcome was recorded.
The treatment group received CC 100 mg per oral once a day, starting day 3 or 5 of menstrual cycle alternately for 3 days and letrozole 5 mg per oral once a day starting day 4 or 6 of menstrual cycle alternately for 3 days. Likewise, the comparison group received letrozole 5 mg per oral once a day, starting day 3 or 5 of menstrual cycle for 5 days. Starting at day 8 to day 10, serial follicle monitoring was performed with the help of TVS. The number of TVS performed for each participant was based upon the growth of follicles and endometrial thickness after sixth day of medication. After the follicle reached maturity, participants were asked to have intercourse to an intent of conceiving in this cycle. If participant was able to conceive, then her pregnancy outcome was monitored through antenatal care during first trimester.
In the absence of conception, another cycle of the treatment was started. Total duration of the intervention was 3 months. Standard operating protocol for the treatment regimen was prepared so as to be consistent for each participant for the treatment [Figure 1]. summarizes the protocol used during the treatment process.

- Consort diagram.
Statistical analysis
Sample size was calculated using Pocock formula for sample size calculation in clinical trials.[18] Total sample size required for the study was 152 cases with 76 in each group (CC–letrozole treatment and letrozole alone comparison group). Additional 10% of the sample participants were recruited into the study to address dropout issue. Thus, total sample size was 170 (85 in each group). Data were entered into excel and then exported to computer package SPSS (IBM SPSS version 23) to clean and statistically analyze the data. Student t test was carried out to compare the difference in the primary outcome. Secondary outcomes were measured using the Chi-squared test. Results are expressed as mean and standard error of mean. Significance level was set at 0.05 and confidence level at 95%.
RESULTS
Total 81 in treatment group and 78 in comparison group were included into the final analysis of the study. Out of 230 assessed for eligibility, 198 participants were recruited into the study. They were randomly assigned into treatment group (combined CC and letrozole regimen) or the comparison group (letrozole alone regimen). Among 99 participants assigned to each group, 4 in the treatment and 5 in the comparison group were lost to follow-up. Among those who completed the treatment regimen, 14 in the treatment and 16 in the comparison groups were excluded from analysis because the blinding of those participants was compromised due to the absence of outcome assessor in certain period of study. The details are provided in Figures 1 and 2.

- Alternate day clomiphene citrate/letrozole stimulation protocol.
Table 1 summarizes the baseline characteristics of the participants in the treatment and comparison groups. There was no statistically significant difference (P > 0.05) between the mean of basic characteristics such as age (years), body mass index (BMI; kg/m2), hirsutism, duration of infertility (years), number of follicles on basal ultrasonography, and endometrial thickness (mm) of the participants among the treatment and comparison groups. Mean age of women in both the groups was less than 30 years with BMI falling under the category of overweight. Pretreatment mean endometrial thickness was less than 8 mm in both the groups. Hirsutism was present in 67.9% in combined therapy group and 66.7% in letrozole only group.

Table 2 summarizes the baseline hormone levels of participants in both groups. Hormones such as FSH, LH, estradiol, and AMH of the participants among the treatment and comparison groups were taken during enrolment. The result shows that there was slightly difference in the mean hormone level in both the groups with no statistical significance.
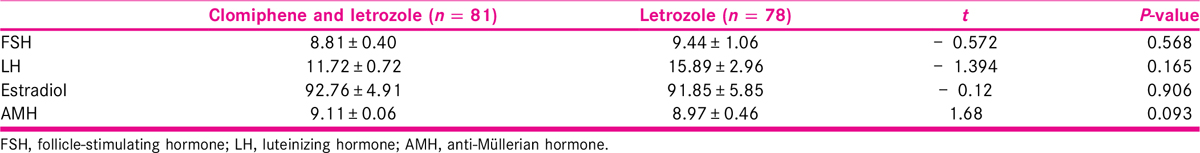
Number of follicles on days 10 to 14 of menstrual cycle of all the participants was measured. As shown in Table 3, we found that the number of matured follicles were slightly higher in the combined CC and letrozole group when compared with letrozole only group (1.59 ± 0.16 in treatment and 1.21 ± 0.56 in comparison, P = 0.03). The difference was statistically significant. Similarly, there was statistically significant difference in the posttreatment mean endometrial thickness among the two therapies (9.04 ± 0.15 in treatment and 8.51 ± 0.12 in comparison, P = 0.01). Likewise, there was statistically significant difference in the mean estradiol level posttreatment with 560.39 ± 9.28 in combined therapy and 513.29 ± 6.94 in letrozole only therapy (P = 0.001). Out of 81 participants, 29 (35.80%) became pregnant in treatment group, whereas 25 participants (32.05%) out of 78 in comparison group became pregnant. This difference was however not significant (χ2= 0.25, P = 0.62). Likewise, 12% in treatment group and 32% in comparison group were found to have miscarriage within the first trimester of pregnancy. This difference was statistically significant (χ2= 3.88, P = 0.05). All the pregnancies were single.
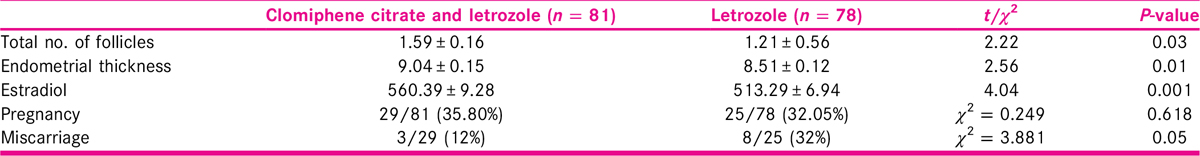
Adverse events occurred during treatment were recorded. No severe adverse events were found during the entire treatment and follow-up period in both the groups. However, among 81 patients who received CC and letrozole, few cases of blurred vision, ankle edema, and nausea were observed which subsided in a day without any treatment. On the contrary, out of 78 participants receiving letrozole only, 2 participants reported of having prolonged bleeding which stopped later on their own.
DISCUSSION
The result showed that both the treatment regimens were able to increase the number of mature follicles and increase the size of endometrium after treatment which is consistent with previous studies.[19] We also observed that the combined therapy was slightly more effective in ovarian induction. As shown by the results, the number of follicles and endometrial thickness increased in combined CC and letrozole group which is similar to the result of previous study where letrozole was associated with lower number of mature follicles per cycle in comparison to CC.[20]
The antiestrogenic effects of CC on endometrium were not observed in the treatment group. There was better endometrial thickness which could be due to higher estrogen availability for endometrial proliferation during multifollicular growth. Hence, even in the CC-resistant PCOS cases, CC works effectively when combined with letrozole alternately. The use of alternate CC during stimulation on endometrium might have lesser antiestrogenic effect on endometrium to favor implantation.
On the contrary, the antiestrogenic effect on hypothalamus and pituitary could have been stronger when used CC and more potent letrozole together facilitating folliculogenesis and steroidogenesis. But we did not measure the FSH and LH levels on the day of HCG to complement our observation as in other studies.[21]
At this point it can be speculated that the combination treatment increased effectiveness of ovulation induction in treatment group when compared with comparison group due to higher estradiol level in the treatment group. Our result is consistent with other studies which showed lower estradiol in letrozole group in comparison with CC group who had significantly higher estradiol level during induction.[13]
The combination of letrozole and CC in women with the CC-resistant PCOS had a statistically higher ovulation rate when compared with those who received letrozole alone in a study. This study had slightly different dose of CC and letrozole given to both the groups. The result from this study is comparable to our study. Our study also supports this result.[22]
Combination of CC and letrozole was beneficial with good results for CC-resistant PCOS cases during induction of ovulation.[15] This study used both CC and letrozole on the same day, whereas our study is the first to known so far to administer the drugs in alternate days and administration for equal days of both the drugs in the same cycle. Our secondary outcome, pregnancy rate was found better in the combined group compared to letrozole alone. This might be the result of shortened time to pregnancy in combined group by increased effectiveness of drugs of different mechanism of action. CC takes longer to clear, because its half-life is about 2 weeks, whereas letrozole has shorter, about 2 days.[23] Thereby, we assumed that the action of CC is slower with release of gonadotropin through H-P-O axis, whereas action of letrozole is faster; it is local inhibition of aromatization and their combined use increased effectiveness of induction to develop better quality oocytes. Of course, more studies needed on this line for appropriate answer.
There are few limitations in our study. First, our study was based only at one center. More such studies are necessary to confirm our doses of these ovulation stimulants for CC-resistant PCOS. Second, we concentrated on low dose longer protocol to avoid possible malformations in the fetuses with higher induction doses. Similar studies with larger sample size and multicentered study are expected to further substantiate our results.
Ethical consideration
Written consent was taken in the consent form developed for the study. The patients were screened for the inclusion criteria during counseling and those who were eligible were invited for the participation in the study. Ethical approval was taken from Nepal Health Research Council before commencement of the study.
Authors’ contribution
The study was conceptualized by Uma Shrivastava and Rabina Dhakal. All authors were involved in designing the study, reviewing literatures, data collection, analysis, and drafting of manuscript. All authors read and approved the final manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Polycystic ovary syndrome. Lancet (London, England) [Internet]. 2007;370:685-97. Available at http://www.ncbi.nlm.nih.gov/pubmed/17720020
- [Google Scholar]
- Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:1-10.
- [Google Scholar]
- Hull and Rutherford classification of infertility. Hum Fertil (Camb). 2002;5(Suppl):S41-5.
- [Google Scholar]
- Treatment strategies for women with WHO group II anovulation: systematic review and network meta-analysis. Bmj [Internet] 2017:j138. Available from: http://www.bmj.com/lookup/doi/10.1136/bmj.j138
- [Google Scholar]
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- [Google Scholar]
- Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction, achievement of pregnancy, and live birth in Asian women with polycystic ovary syndrome: a randomized controlled trial. Fertil Steril. 2009;91:514-21.
- [Google Scholar]
- Comparison of the efficiency of clomiphene citrate and letrozole in combination with metformin in moderately obese clomiphene citrate-resistant polycystic ovarian syndrome patients. Srp Arh Celok Lek. 2016;144:146-50.
- [Google Scholar]
- Combined metformin and clomiphene citrate versus laparoscopic ovarian diathermy for ovulation induction in clomiphene-resistant women with polycystic ovary syndrome: a randomized controlled trial. J Obstet Gynaecol Res. 2011;37:169-77.
- [Google Scholar]
- Comparison of two aromatase inhibitors in women with clomiphene-resistant polycystic ovary syndrome. Int J Gynaecol Obstet. 2004;85:289-91.
- [Google Scholar]
- Comparison of efficacy of aromatase inhibitor and clomiphene citrate in induction of ovulation in polycystic ovarian syndrome. Fertil Steril. 2009;92:853-7.
- [Google Scholar]
- Combined clomiphene citrate-metformin versus letrozole-metformin in achieving pregnancy among women with polycystic ovary syndrome. Gynecol Reprod Health. 2019;3:1-5.
- [Google Scholar]
- Clomiphene citrate or letrozole for ovulation induction in women with polycystic ovarian syndrome: a prospective randomized trial. Fertil Steril. 2009;92:849-52.
- [Google Scholar]
- Comparison of efficacy of letrozole and clomiphene citrate in ovulation induction in Indian women with polycystic ovarian syndrome. Arch Gynecol Obstet. 2012;285:873-7.
- [Google Scholar]
- Combined letrozole and clomiphene versus letrozole and clomiphene alone in infertile patients with polycystic ovary syndrome. Drug Des Devel Ther. 2013;7:1427-31.
- [Google Scholar]
- Simultaneous clomiphene citrate and letrozole therapy for ovulation induction in clomiphene-resistant polycystic ovarian syndrome. Fertil Steril [Internet]. 2013;99:S36. Available at https://doi.org/10.1016/j.fertnstert.2013.01.082
- [Google Scholar]
- Infertility: an emerging public health issue in Nepal. Ann Clin Chem Lab Med. 2017;3:1-2.
- [Google Scholar]
- What is the best treatment option for infertile women aged 40 and over?. 2013;30:667-71.
- Clinical Trials: A Practical Approach. Chichester [West Sussex]; New York: Wiley; 1983.
- Letrozole versus combined metformin and clomiphene citrate for ovulation induction in clomiphene-resistant women with polycystic ovary syndrome: a randomized controlled trial. Fertil Steril. 2010;94:1405-9.
- [Google Scholar]
- Meta-analysis of letrozole versus clomiphene citrate in polycystic ovary syndrome. Reprod Biomed Online. 2011;23:91-6.
- [Google Scholar]
- Comparison of the use of letrozole and clomiphene citrate in regularly ovulating women undergoing intrauterine insemination. Middle East Fertil Soc J. 2006;11:113-8.
- [Google Scholar]
- A randomized controlled trial of combination letrozole and clomiphene citrate or letrozole alone for ovulation induction in women with polycystic ovary syndrome. Fertil Steril. 2019;111:571-578.e1.
- [Google Scholar]
- Letrozole or clomiphene citrate as first line for anovulatory infertility: a debate. Reprod Biol Endocrinol [Internet]. 2011;9:86. Available at https://doi.org/10.1186/1477-7827-9-86
- [Google Scholar]







