Translate this page into:
A Review on the Role of Endometrial Microbiome in Reproductive Pathologies Affecting Female Infertility

*Corresponding author: Sayali Kandari, Department of Reproductive Medicine, Cellsure Biotech Research Centre, Maharashtra, India. drsayalikandari@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kandari S. A Review on the Role of Endometrial Microbiome in Reproductive Pathologies Affecting Female Infertility. Fertility Science and Research. 2024;11:5. doi: 10.25259/fsr_43_23
Abstract
Infertility is a disease with a complex pathophysiology and concurrent presence of gynaecological and reproductive disorders associated with it. A significant hurdle that infertility faces is the limitation of the success rate per couple per cycle in one assisted reproductive technology treatment. A central role is played by the complex embryo endometrial crosstalk that has been a subject of study since the very beginning of natural conception failures. The endometrium is a key player in the attempt for a healthy live birth. Thought to be previously sterile, the uterus is now known as home to a unique community of microorganisms. The microbiota and their genomic content: the endometrial microbiome is a complex and heterogeneous endocrine system that plays a pivotal role in the master orchestration of successful embryo implantation in a receptive uterine cavity. The microbial community inside the uterus is now known to influence reproductive pathologies and complex aetiologies that influence infertile outcomes. This review is aimed to understand the correlations of the endometrial microbiome and more specifically, bacterial communities and their pathophysiology in reproductive pathologies leading to infertility. The role of personalised regimens and therapies for treatment through nutritional, microbiotic or pharmaceutical interventions is discussed. The use of selected strains that are part of this bacterial community as probiotics could be a successful therapy for uterine diseases and concomitant infertility alongside nutritional and pharmaceutical interventions. Further longitudinal studies in infertile patients with specific pathologies with stratified selection are necessary to progress microbiome evaluation and eventually, modulation for infertility.
Keywords
endometrial microbiome
uterine microbiome
infertility
probiotics
chronic endometritis
endometriosis
polycystic ovarian disease
endometrial cancer
endometrial hyperplasia
microbiota
microbiome composition
INTRODUCTION
Infertility is a systemic disease with huge public, familial and countrywide social and economic implications. The current focus of infertility is on improving outcomes per assisted reproductive technology (ART) cycle and customisation of diagnostics and treatment to optimise per couple and cycle pregnancy rates. In this endeavour, many multidisciplinary approaches have been attempted, and newer technologies and techniques have been executed to improve outcomes in subfertile patient groups.
The human microbiome is quantified as the entirety of microorganisms and their genomes inhabiting the human body. Understanding the role of this large entity encompassing the microbiome in both physiologic and pathophysiologic reproductive processes has begun with numerous research papers. Culture technologies have severe limitations and newer genomic technologies using high-throughput sequencing technologies have characterised the human microbiome in normal, healthy volunteers at different body sites.[1]
The technologies from the field of metagenomics revealed surprising information that sites in the body historically thought to be completely sterile, like the uterine cavity or the placenta, have their unique microbiome. Sequencing methods involve the 16S rRNA gene unique to the bacterial genome and provide reliable results to enhance diagnosis and treatment alongside classic methods.[2]
Multiple studies try to explain the origin of the endometrial microbiota. A study says spermatozoa introduce microorganisms in the female genital tract (FGT),[3] whereas other studies suggest the use of contraceptives or ART procedures.[4] The most popular and supported theory is that uterine microbial colonisation occurs through vaginal ascension, oral cavity, gut and bloodstream.[5] There is fair debate as to the composition of uterine microbiota, but they certainly exist.[6]
METHODOLOGY
The methodology and writing parameters are followed as proposed under SANRA, a quality assessment scale.[7] This review article aimed to address the effects of the endometrial microbiome in the context of the pathology of infertility and associated gynaecological conditions like chronic endometritis (CE), endometriosis and polycystic ovary syndrome (PCOS). A literature search was conducted using search engines like ‘Google Scholar’ and ‘PubMed’ to obtain relevant articles to determine the effect of microbiome on fertility. The keywords used to generate the search included ‘endometrial microbiome’, ‘vaginal microbiome’, ‘endometrial dysbiosis’, ‘Lactobacillus’ and ‘gut microbiome’. Articles included in this manuscript were open access and not restricted to any dates. The research articles that fit the inclusion criteria were screened for relevance and added to the manuscript. A manual search of the references within the obtained articles was then performed and added based on relevance to the topic of this article. The selected articles were prepared by three research associates and further screened for relevance by the invited author. Structured headings corresponding to the subject matter and each relevant pathology are mentioned in scientific terms to assist the audience.
ENDOMETRIAL MICROBIOME – COMPOSITION
The endometrial microbiome is by no means a stable entity in the uterus. It changes across the lifespan of a woman and is influenced by age, sexual activity, contraceptives and any infections or dysbiosis of the gut and oral microbiome [Figure 1].[6]
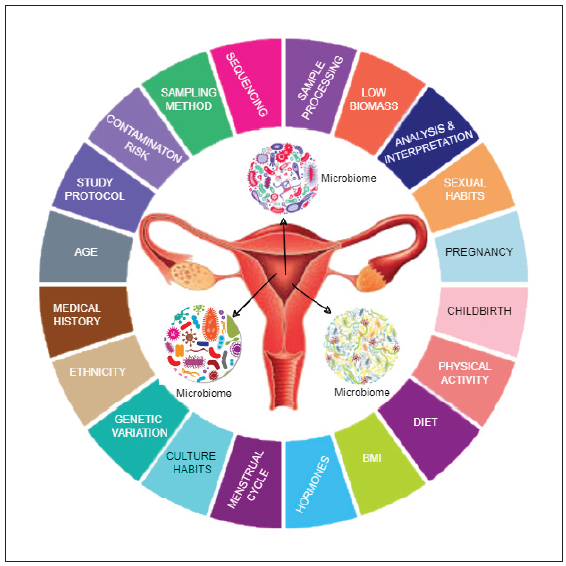
- The endometrial microbiome influences and is influenced by a myriad of factors: genetic, environmental, metabolic, age and the interventions in the affected female. Infertility as an outcome needs multidisciplinary intervention in the context of microbiota modulation as a therapeutic tool in ART. BMI: Body mass index, ART: Assisted reproductive technology.
Fecundity and gravida status, including the number and route of deliveries (caesarean vs vaginal), influence the microbiome composition in the FGT. Further hormonal changes impact and change the composition, and it is noted that the microbiome potentially changes throughout the menstrual cycle of a woman making its molecular signature unique to the time of implantation.[8] This is an important and attractive therapeutic target, considering that the increasing role of the microbiome is being revealed to play in the master orchestration of successful embryo implantation in a receptive endometrium.[9]
The microbiome composition has been mapped by multiple research groups with varying results. The current understanding and important microbial species identified in endometrial samples are Lactobacillus spp., Gardnerella, Anaeroccocus, Bifidobacterium, Atopobium, Prevotella and Streptococcus species. This is by no means an exhaustive list. In fact, in a study on Chinese women, the microbiome was dominated by Acinetobacter, Pseudomonas, Sphingobium and Vagococcus.[10]
In a prospective cohort study of a small sample of 30 women in a single fertility clinic undergoing frozen embryo transfer (FET), endometrial microbiome analysis was conducted in all patients and results between pregnant and non-pregnant women were compared. Certain species of bacteria were more abundant in non-pregnant women, with an emphasis on the higher presence of Bacteroides and an increase in Serratia marcescens and Staphylococcus aureus. In contrast, Bosea species were found only in the pregnant group. Alpha and beta diversity between both groups was not different. Surprisingly, no differences were observed in the concentration of Lactobacillus species in both groups, which is a contrasting finding to other studies.[11]
An observational study evaluated the microbiome by evaluating tips of embryo transfer catheters from 70 women who displayed a marked abundance of Lactobacillus in all the samples (50 patients had over 70% Lactobacillus).[12] A review of 13 fertile women showed Lactobacilli dominance in endometrial fluid.[13]
Overall, the diversity, presence and commensal status of bacterial species is a subject under evaluation; in different pathologies, patient groups, ages, diagnostic assays and clinical modalities should follow with correlating classic diagnostic criteria with sequencing results.
THE DYSBIOTIC ENDOMETRIAL MICROBIOME
To explain the pathophysiology of a disturbed endometrial microbiome, formally known as endometrial microbiome dysbiosis, various proposed mechanisms are discussed in an attempt to explain how changes in the microbial community inside the uterine cavity influence infertility, gynaecological pathologies and obstetric outcomes.
Maintenance of vaginal microbiota through certain bacteria is well documented. Lactobacillus spp. plays an important role via the production of bacteriocins, hydrogen peroxide and lactic acid, lowering vaginal pH and preventing the growth of pathogenic bacteria. A further theory suggests competitive adhesion by commensal microbes to the vaginal epithelium and immune response modulation creates a hostile environment for pathogenic bacteria supporting genital tract health.[7]
If such is the case, why is the dysbiotic state of the uterus tolerated by the physiology of women suffering from its effects? The reason is that virulence mechanisms such as antimicrobial resistance, mucin degradation and biofilm creation cause balanced dysbiotic states.[14]
A hypothesis of endometrial pH distortion leading to dysbiosis is suggested by a few groups. One hypothesis postulates that a decrease in quantifiable Lactobacilli would increase FGT pH, eventually affecting endometrial pH, impacting embryo attachment ability and allowing the growth of pathogenic microbial community leading to infertility.[2]
However, another group, having tested endometrial pH in an endometrial fluid sample of 14 patients, demonstrated the same range of pH values regardless of the bacterial composition, suggesting a more complex mechanism beyond pH changes.[13]
Another hypothesis is the vaginal ascension of microorganisms. It has been demonstrated that the endocervical barrier can be broken easily. A study introduced radioactively labelled macrospheres through the external cervical os deposition, and within minutes was shown to have ascended in the uterine cavity.[6] Further, this ascent is faster, seen mainly during the follicular and luteal phases of the cycle, establishing the feasibility of bacterial seeding of the uterine microbiome via the vaginal route.[15]
The hypothesis most supported by literature is the gut reproductive axis. The gut microbiome has been mapped and referenced most commonly due to its effect on multiple disease pathologies, including metabolic disorders and chronic diseases. Studies have stated the interplay between the estrogen-gut axis and cancer. It has been shown that the microbes found in the gut cause the reutake of estrogen which enhances cancers driven by estrogen.[15] Patients with breast cancer have different estrogen levels and different populations of gut microbiota when compared to healthy individuals. Furthermore, via the “estrobolome,” gut microbial populations secrete β-glucuronidase which modulates the concentration of free estrogen, by deconjugation of estrogen into its active metabolites.[15,16]
These studies further cement the basis of hormonal regulation via gut microbiome as an endocrine organ separate from the identified ones. Endometrial fluid samples in 110 patients undergoing fertility treatment demonstrated upregulation of factors correlated to cell adhesion, immunological response and inflammation.[17]
Endometrial microbiome correlation to infertility is increasingly displayed. Further, the microbiome of the lower FGT has been established to play a pivotal role in many reproductive pathologies, where a large correlation and incidence of the same exists in female infertility with expressions of immunoglobulins IgM, IgA and IgG in patients with CE and recurrent implantation failure (RIF) different than non-CE patients,[18] and increase in pro-inflammatory cytokines interleukin 6 (IL-6), proIL-1β and IL-1β are seen in endometriosis.[19]
It is well worth studying their effects jointly on the presentation of female endometrial infertility.
Endometrial Microbiome and Infertility with Different Reproductive Pathologies
Infertility is characterised by endometrial and female reproductive and genital tract abnormalities that further influence female reproductive outcomes.
Recent studies show that uterine, cervical and vaginal microbiota influence CE, polyposis, myomatosis, polycystic ovarian syndrome (PCOS) and urogenital tract infections. Reproductive microbiota also influences infertility issues like RIF, recurrent miscarriages (RMs) and endometrial receptivity [Figure 2].[14]
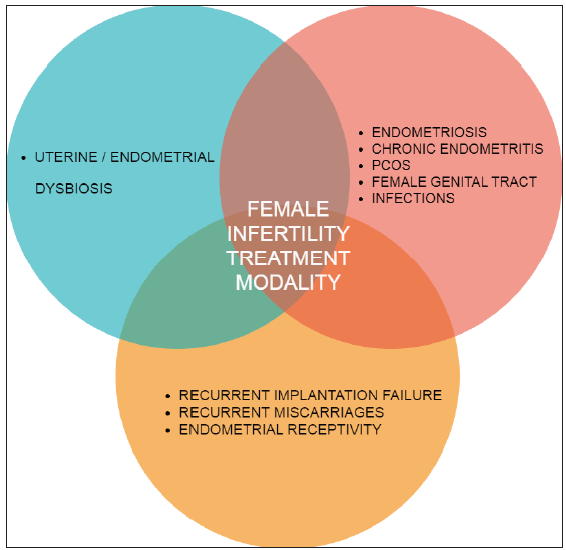
- Endometrial Microbiome Dysbiosis has a large role in gynaecological disorders and concurrently is seen present in female infertility, specifically in RIF, RM cases as well as impaired endometrial receptivity. All three of these issues interact and influence each other in the above Venn diagram affecting female treatment for infertility modality in ART. RIF: Recurrent implantation failure, RM: Recurrent miscarriages, ART: Assisted reproductive technology.
CHRONIC ENDOMETRITIS
CE is treated to modulate the endometrial microbiome with hysteroscopy findings, histology and CD138+ plasma cell infiltration assessment. Evaluation methods in this observational study are in two different settings as distension fluid used for hysteroscopy affects microbiologic investigations. Post the hysteroscopy the endometrial sampling is performed during the follicular phase of the following cycle.[20]
In a case series, patients with presence of gram-negative bacteria have been prescribed treatment with 500 mg ciprofloxacin twice a day for 10 days, which therefore constitutes the first-line therapy. For gram-positive bacteria, 1 g amoxicillin þ clavulanate twice a day is prescribed for 8 days. Mycoplasma, Ureaplasma urealyticum, and Chlamydia are treated with 1 g josamycin twice a day for 12 days, and 100 mg doxycycline or minocycline twice a day for 12 days is used when the infection persists. Negative cultures with positive endometritis picture of the ovary with pale cavity and lesions are treated with 250 mg ceftriaxone intra-muscularly in a single dose plus 100 mg doxycycline orally twice a day, and 500 mg metronidazole orally twice a day for 14 days. This is in agreement with Centers for Disease Control and Prevention guidelines for treating infections caused by anaerobes. If signs of CE persist (positive culture and/or histology) after therapy, the treatment protocol is advised three times. Once both are negative, a final hysteroscopy confirms the normalization of the endometrium. In a study on 48 RIF patients, this protocol resolved infertility in 45 patients.[20,21]
Resistant CE is further treated on a case-by-case basis with intrauterine infusion of antibiotic cocktails designed to work against the putative cultured bacteria. This approach is novel and warrants further study and evaluation.[21]
Combined pathologies also pose a challenge while treating infertility. Higher levels of Lactobacillus in diseased groups of women with endometrial polyps (EP) or in women with EP and chronic endometritis (EP + CE) (38.64 and 33.21%, respectively) compared with healthy controls (6.17%) report by Fang et al. in a review article.[22]
Infertility association with CE is well documented. In endometrial biopsies and fluid from 130 infertile patients in an observational study, a lower abundance of Lactobacilli and higher quantification of Anaerococcus, Bifidobacterium, Dialister, Gardnerella and Prevotella was demonstrated.[20]
In 60 patients undergoing IVF treatment, a decrease in Lactobacillus spp. abundance among CE patients with a higher abundance of Ralstonia spp. and Gardnerella spp. were associated with CE.[23]
Studies showed higher pregnancy rates and live birth rates (LBRs) in women with CE after successful treatment with antibiotics, establishing the importance of microbiome modulation in endometritis.[20]
ENDOMETRIOSIS
Antibiotics given to women with endometriosis significantly increased their implantation outcomes compared to those women who were not administered antibiotics. This could suggest that the presence of uterine bacteria could attribute to the harmful effects on reproductive outcomes in women with endometriosis.[20]
Colony-forming units (CFUs) of Streptococcus, Gardnerella, and Enterococcus, were increased in cultured samples of 73 women with endometriosis when compared to the control group of 55 women. While 64 women with endometriosis had lower uterine Lactobacilli abundance and increased Streptococcaceae and Moraxellaceae abundance.[24]
Another study found that Gardnerella, Lactobacillus, Prevotella, and Streptococcus was observed in both the control and endometriosis samples, which suggests that there is no change to the microbial population between both groups. However, the assessment showed that deep endometriotic lesions have increased populations of Enterococcus, Pseudomonas, and Alishewanella, and there is a significant microbiome shift is seen in stage III endometriosis.[25]
Success in identifying unique species in the uterine microbiome that are associated with a particular disease state could potentially be used as microbial biomarkers for prevention, screening, diagnosis or even treatment to improve health and reproductive success.
POLYCYSTIC OVARY SYNDROME
PCOS is a complex and heterogeneous endocrine disease. The hypothesis of the endometrial and genital tract microbiome involved in the development of PCOS has been put forth.
Much evidence has focused on sex hormones and insulin-resistance correlation to intestinal microbiota, including hyperandrogenism aetiology and chronic low-grade inflammation. The variations are attributed to inflammatory cytokines modulating insulin and role gastrointestinal hormones Ghrelin, peptide YY (PYY), bile acids, interleukin-22 and microbes Bacteroides vulgatus.[26]
PCOS has been demonstrated to be permissible to modulation with implantation regulating small molecules like hyaluronan, with various studies showing improvement in pregnancy rates. Kandari et al. conducted a randomised controlled trial showing improved LBRs and reduced miscarriage rates in fresh transfers in the PCOS group, demonstrating the malleability of the implantation event.[27] Therapeutic modulation in the future could potentially include probiotics, prebiotics, synbiotics, faecal microbiota transplantation FMT, and IL-22 usage, among others, as a modulator for the microbiome.[26,28]
MICROBIOME IN OTHER COMPLEX REPRODUCTIVE PATHOLOGIES AND INFERTILITY
Bacterial Vaginosis (BV) is correlated to various lower genital tract abnormalities and is associated with pelvic inflammatory disease, and CE also shows an abundance of Gardnerella vaginalis, Atopobium vaginae and Prevotella bivia.[28]
ENDOMETRIAL MICROBIOME AND INFERTILITY OUTCOMES
Currently, loss of pregnancy, RIFs and Reviewer 2 correction 4: repeated miscarriages are huge burdens on clinical providers and patients in the infertility service provision. While pre-implantation genetic testing and time-lapse imaging morphometry have provided a higher efficiency in selecting high-quality embryos, the endometrial equation is still fraught with several inefficiencies. There are immunological factors, uterine abnormalities or the presence of endometrial pathologies that reduce outcomes and healthy babies born per ART treatment commenced. Endometrial evaluation is a critical issue in blastocyst implantation and is now a routine assessment in RIF and RMs.[29]
Along with classical diagnostic tools such as hysteroscopy, vaginal and cervical evaluations for infectious agents and endometrial microbiome evaluation are being studied in clinical research settings as potential biomarkers to diagnose uterine diseases and the use of antibiotic probiotic combinations in the treatment algorithm.
REPEATED IMPLANTATION FAILURE
Research groups have been working on the aetiology of RIF since the beginning of ART being used in infertile patients. A strong link has been identified in investigating the effects of endometrial microbiota on RIF.
Forty-six RIF patients attempting IVF for the first time were evaluated for microbial analysis in endometrial fluid. There was a decrease in microbial diversity in the endometrial fluid in RIF patients.[18] Dominant bacteria L. helveticus, Sneathia amnii and the genus Prevotella were reported abundant in RIF patients while higher abundance L. iners, L. jensenii and genus Ralstonia were found in subjects without RIF.[30]
Restoring Lactobacilli abundance via the gut and vaginal route are common modalities of treatment. In one such observational study where LD status was attempted to be restored, patients with Gardnerella as dominant bacteria had decreased response to modulation.[30]
RECURRENT MISCARRIAGES
RMs, including biochemical pregnancies and first trimester loss, plague the vision of delivering a healthy baby which is the desired outcome of ART. Due to the incidence and adverse outcomes arising from pregnancy loss, ART treatment success is currently limited by incomplete knowledge of the aetiology of RM.
Clinical miscarriages are associated with women who lack a Lactobacillus dominant profile, but have an increased number of other bacterial species. There is a reduced amount of bacterial species in women during their fourth gestational week.[31]
A prospective observational multicenter study was conducted in 342 women that showed that Lactobacillus was abundant in women with ongoing pregnancy while presence of Neisseria, Haemophilus, Gardnerella, Streptococcus, Bifidobacterium, Klebsiella, Atopobium, Staphylococcus, and Chryseobacterium was increased and had adverse early pregnancy outcomes, namely the absence of pregnancy, biochemical pregnancies, or miscarriages. Further, biochemical pregnancies are associated with higher Enterococcus abundance, while Klebsisella, Gardnerella and Streptococcus were higher in clinical miscarriage.[6]
ENDOMETRIAL RECEPTIVITY
Endometrial receptivity is a large concern and is considered a contributing factor to RIF and also a causal factor in RM and adverse obstetric outcomes like abnormal placentation and even preeclampsia. It is quite evident that there are different degrees of endometrial receptivity, some that allow implantation but or impair growth, some that support growth but disturbs placentation and have a role in determining vaginal versus caesarean birth result of fertility treatment.[32]
Diagnostic criteria such as gene expression arrays or sequencing are used on a large scale in fertility treatments with mixed results. One reason is the complex and unexplained aetiology of receptivity and the role of microbiome dysbiosis in its clinical presentation.[33]
Uterine microbiome and the correlation of endometrial receptivity have begun in earnest. Currently, although associations are not universal, it is now being accepted that microbiome evaluation may need to be studied and corrected before endometrial receptivity evaluation as current treatment modalities change endometrial receptivity in treated patients.[34]
The endometrial microbiomes’ role in embryo endometrial crosstalk should be evaluated as the implantation event is characterised by highly significant changes in pro-inflammatory cytokines and permissibility of the uterine submucosal layer to the embryo invasion.[35]
The next phase of clinical research hopes to study endometrial receptivity in infertility as well as early pregnancy to unravel the methodology of its expression in achieving and maintaining pregnancy till term.
TREATMENT MODALITIES IN ENDOMETRIAL MICROBIOME DYSBIOSIS
This review aims to understand the correlations of the microbiome and, more specifically, bacterial communities and their pathophysiology in reproductive pathologies and complex aetiologies that influence infertility.
The goal, it appears, is that of a physiological endometrial microbiota agreeable to embryo implantation. Beyond diagnostic tools, the role of personalised regimens and therapies for infertility treatment through nutritional, microbiotic or pharmaceutical interventions is discussed.
The first line of therapy in disrupting the pathology of endometrial dysbiosis has been antibiotic therapy. This has now further progressed to using antibiotic and probiotic supplementation via the oral route and vaginal route. Combination therapies utilising specific bacterial strains in infertility diagnosis are now routine but not based on robust clinical evidence.[36]
Most therapies have been designed for the post-empirical treatment of personal infertility practices, and no consensus currently exists on the right dosage or bacterial strain combinations that provide benefits in specific reproductive pathologies.[37] Currently, multiple studies have been designed to answer these questions in infertile women with and without concomitant gynaecological disorders like CE, endometriosis and polycystic ovary disease.
Other modalities include vaginal microbiota transplant or FMT; however, both methodologies are currently fraught with a high risk of infectious disease transfer, and FMT is only licensed for Clostridium difficile infections.[38]
In treated cell cultures strains of Bifidobacterium and Lactobacillus species decreases the pH and prevents the survival of pathogenic bacteria such as Streptococcus agalactiae and P. acnes, and further reduce the inflammatory effects induced by these pathogens by lowering the levels of MCP-1, IL-6, and IL-8 by releasing short-chain fatty acids.[36]
To treat inflammatory uterine diseases, treatment could be in the form of administering probiotics or performing endometrial microbiota transplantation. Focus should given to the extent to which endometrial microbes influence implantation, and how uterine dysbiosis changes their micro-environment.
DISCUSSION
ART clinical outcomes are influenced by all the uterine microbial communities and their systemic interaction with associated tissues. Defining a healthy endometrial microbiome is not just about the abundance of certain genera, but also the correlative communities that are supportive of embryo implantation and pregnancy continuity until birth. Some pathogenic bacteria are shown to be tolerable to the endometrium, but the correlative immune factors, as well as the microbiome community balance, should be within the range which is under evaluation and study.
Further studies should focus on a multidisciplinary algorithm in detecting and evaluating microbiome status alongside immunological profile, hysteroscopy, anatomical and histopathology findings and a detailed history and modification of diet and lifestyle factors that influence the gut microbiome axis that is implicated in genital tract microbiome modulation along with the endometrial microbiome. Such studies will provide proper guidance to the clinical community as to how to handle the issue of microbiome modulation in reproductive pathologies leading to infertility.
CONCLUSION
Multiple studies have shown that the reproductive microbiome acts as a complex web composed of different microorganisms which plays a crucial role in female infertility. Any alterations to the reproductive microbiome are associated with a plethora of gynaecological diseases. Proper modulation of the reproductive microbiome may prove to be beneficial and aid in the treatment of female infertility thereby improving clinical pregnancy rates and live birth rates.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Potential Influence of the Microbiome on Infertility and Assisted Reproductive Technology. Semin Reprod Med. 2014;32:035-42.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Endometrial Microbiome: Sampling, Assessment, and Possible Impact on Embryo Implantation. Sci Rep. 2022;12:8467.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mycoplasma Genitalium Attaches to Human Spermatozoa. Hum Reprod. 2003;18:2103-9.
- [CrossRef] [PubMed] [Google Scholar]
- Identifying the Epigenetic Basis of Idiopathic Infertility using Next-generation Sequencing of Spermatozoal RNAs. Fertility and Sterility. 2016;105
- [CrossRef] [Google Scholar]
- Uterine Microbiota: Residents, Tourists, or Invaders? Front Immunol. 2018;9
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The Endometrial Microbiome and its Impact on Human Conception. Int J Mol Sci. 2022;23:485.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res Integr Peer Rev. 2019;4:5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- What Fertility Specialists should know about the Vaginal Microbiome: A Review. Reprod Biomed Online. 2017;35:103-12.
- [CrossRef] [PubMed] [Google Scholar]
- Biologia Futura: Endometrial Microbiome Affects Endometrial Receptivity from the Perspective of the Endometrial Immune Microenvironment. Biol Futur. 2022;73:291-300.
- [CrossRef] [PubMed] [Google Scholar]
- The Microbiota Continuum along the Female Reproductive Tract and its Relation to Uterine-related Diseases. Nat Commun. 2017;8:875.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association between Endometrial Microbiome and Implantation Success in Women with Frozen Embryo Transfer: Results of a Prospective Cohort Study. Biotechnol Biotechnol Equip. 2023;37:1.
- [CrossRef] [Google Scholar]
- Characterizing the Endometrial Microbiome by Analyzing the Ultra-low Bacteria from Embryo Transfer Catheter Tips in IVF Cycles: Next Generation Sequencing (NGS) Analysis of the 16S Ribosomal Gene. Hum Microbiome J. 2017;3:15-21.
- [Google Scholar]
- Evidence that the Endometrial Microbiota has an Effect on Implantation Success or Failure. Am J Obstet Gynecol. 2016;215:684-703.
- [CrossRef] [PubMed] [Google Scholar]
- Current Findings in Endometrial Microbiome: Impact on Uterine Diseases. Reproduction. 2022;163:R81-96.
- [CrossRef] [PubMed] [Google Scholar]
- Estrogen – Gut Microbiome Axis: Physiological and Clinical Implications. Maturitas. 2017;103:45-53.
- [CrossRef] [PubMed] [Google Scholar]
- Endometrial Microbiome. Curr Opin Obstet Gynecol. 2017;29:146-52.
- [CrossRef] [PubMed] [Google Scholar]
- Differential Proteomic Analysis of Endometrial Fluid Suggests Increased Inflammation and Impaired Glucose Metabolism in Non-implantative IVF Cycles and Pinpoints PYGB as a Putative Implantation Marker. Hum Reprod. 2018;33:1898-906.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive Endometrial Immunoglobulin Subclass Analysis in Infertile Women Suffering from Repeated Implantation Failure with or without Chronic Endometritis. Am J Reprod Immunol. 2014;72:386-91.
- [CrossRef] [PubMed] [Google Scholar]
- Association of the Precursor of Interleukin-1β and Peritoneal Inflammation Role in Pathogenesis of Endometriosis. J Clin Lab Anal. 2016;30:831-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of Chronic Endometritis in Repeated Unexplained Implantation Failure and the IVF Success Rate After Antibiotic Therapy. Hum Reprod. 2014;30:323-30.
- [CrossRef] [PubMed] [Google Scholar]
- Efficient Treatment of Chronic Endometritis Through a Novel Approach of Intrauterine Antibiotic Infusion: A Case Series. BMC Womens Health. 2018;18:197.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- How Uterine Microbiota Might be Responsible for a Receptive, Fertile Endometrium. Hum Reprod Update. 2018;24:393-415.
- [CrossRef] [PubMed] [Google Scholar]
- Endometrial Microbiome: New Hope, or Hype? Reprod Biomed Online. 2021;42:1051-2.
- [CrossRef] [PubMed] [Google Scholar]
- Endometrial Microbiota—New Player in Town. Fertil Steril. 2017;108:32-9.
- [CrossRef] [PubMed] [Google Scholar]
- Microbiome Profifile of Deep Endometriosis Patients: Comparison of Vaginal Fluid, Endometrium and Lesion. Diagnostics. 2020;10:163.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Microbiome and PCOS: State-of-art and Future Aspects. Int J Mol Sci. 2021;22:2048.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Time Lapse Selected Elective Single Embryo Transfer in Hyaluronon Enriched Transfer Medium in PCOS Improves Live Birth Rates Compared to Use of Conventional Embryo Transfer Media. A Possible Alternative to Freeze-all Cycles in PCOS. Fertil Steril. 2019;112:e47-8.
- [CrossRef] [Google Scholar]
- Investigating the Effect of an Abnormal Cervico-Vaginal and Endometrial Microbiome on Assisted Reproductive Technologies: A Systematic Review. Am J Reprod Immunol. 2018;80:e13037.
- [CrossRef] [PubMed] [Google Scholar]
- New Opportunities for Endometrial Health by Modifying Uterine Microbial Composition: Present or Future? Biomolecules. 2020;10:593.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Characterization of the Endometrial Microbiome in Patients with Recurrent Implantation Failure. Microorganisms. 2023;11:741.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Optimal Uterine Anatomy and Physiology Necessary for Normal Implantation and Placentation. Fertil Steril. 2016;105:844-54.
- [CrossRef] [PubMed] [Google Scholar]
- Prenatal and Postnatal Determinants in Shaping Offspring’s Microbiome in the First 1000 days: Study Protocol and Preliminary Results at One Month of Life. Ital J Pediatr. 2020;46:45.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mapping the Entire Functionally Active Endometrial Microbiota. Hum Reprod. 2021;36:1021-31.
- [CrossRef] [PubMed] [Google Scholar]
- Uterine Microbiome and Endometrial Receptivity. Ceska Gynekol. 2019 Winter;84:49-54. English. PMID: 31213058
- [PubMed] [Google Scholar]
- Cysteine Dependence of Lactobacillus Iners is a Potential Therapeutic Target for Vaginal Microbiota Modulation. Nat Microbiol. 2022;7:434-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Patent Watch: Modulating the Human Microbiome with Live Biotherapeutic Products: Intellectual Property Landscape, Nature Reviews. Drug Discov. 2016;15:224-5.
- [CrossRef] [PubMed] [Google Scholar]
- The Impact of the Female Genital Tract Microbiome in Women Health and Reproduction: A Review. J Assist Reprod Genet. 2021;38:2519-41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







