Translate this page into:
Role of genetics, environmental, and lifestyle factors responsible for infertility − A review
Address for correspondence: Dr. Rakesh Kumar, Shri Mata Vaishno Devi University Campus, Sub-Post Office, Katra, Jammu and Kashmir – 182320, India. E-mail: kumar.rakesh@smvdu.ac.in
-
Received: ,
Accepted: ,
How to cite this article: Sharma M, Sharma B, Jamwal RS, Shah R, Kumar R. Role of genetics, environmental, and lifestyle factors responsible for infertility - A review. Fertil Sci Res 2023;10:131-8.
Abstract
Infertility is characterized by the inability to achieve pregnancy even after engaging in regular and unprotected sexual intercourse for a minimum duration of 1 year. Infertility is a serious condition affected by various lifestyle and environmental factors. Ranked as the fifth most significant disability globally, its prevalence is steadily escalating with each passing day. Approximately 60 to 80 million of couples in reproductive age are infertile. In India, primary infertility occurs at a rate ranging from 3.9% to 16.8%. Within the region of Jammu and Kashmir, the prevalence of infertility stands at 15%, with an even higher prevalence of 15.7% specifically in the Kashmir area. Human reproduction is very susceptible to genetics, environmental, and lifestyle changes. Infertility can be significantly influenced by genetics. Infertility in men and women can be brought on by genetic issues like chromosomal abnormalities, gene mutations, and epigenetic modifications. In rare situations, parents with gene mutations that impact reproductive function can pass on sterility to their offspring. For instance, infertility is linked to some genetic illnesses such as Turner syndrome, Klinefelter syndrome, and Fragile X syndrome. Environmental factors and lifestyle factors can affect the fertility of both males and females. Due to this reason, human fertility is decreasing for the last 50 years. The lifestyle and environmental factors are the modifiable habits that actually influence overall human health. The objective of this review is to ascertain the influence of environmental and lifestyle elements on reproductive well-being. A multitude of investigations and scholarly inquiries within this domain have increasingly highlighted the significant repercussions of lifestyle choices and environmental conditions on fertility.
Keywords
Diet
environment
genetics
infertility
lifestyle
INTRODUCTION
Infertility stands as a prominent challenge within the realm of gynecology, constituting a global issue recognized by the World Health Organization (WHO).[1] Infertility is a medical condition that pertains to the inability of an individual or a couple to achieve conception despite engaging in consistent, unprotected sexual activity.[2] Infertility presents in various forms, each highlighting distinct underlying causes and challenges. Primary infertility characterizes couples who are unable to conceive their first child despite consistent efforts. Secondary infertility pertains to those who face difficulty conceiving subsequent children after having previously experienced successful pregnancies. This issue is not restricted to either gender; rather, it can arise from various factors affecting both men and women. In case of women, causes of infertility can encompass irregular ovulation patterns, obstructions in the Fallopian tubes, uterine abnormalities, endometriosis, and hormonal imbalances. In men, infertility might result from complications in sperm production, function, or delivery. These complications could manifest as low sperm count, poor sperm motility, or structural irregularities. In certain instances, infertility may emerge due to a combination of factors originating from both partners.[3,4] Infertility is a polygenic disorder, including various genes, interactions between genes, and correlations between genes and environment, all of which affect how infertility develops. Additionally, there are cases where despite comprehensive medical evaluation, the precise cause of infertility remains unidentified. Lifestyle choices can also significantly influence fertility. Advanced age, excessive body weight, smoking, alcohol consumption, recreational drug use, and exposure to environmental pollutants can all impact the ability to conceive in both men and women.[5,6]
Incidence of infertility
Tang et al. studied that almost 10% to 15% of couples of reproductive ages have defective fertility, and in 50% of these cases, the male factor is responsible.[7] In the recent years, the prevalence of infertility is increasing with the increased use of assisted reproductive technologies (ART) analyzed by Katole et al.,[5] their investigation revealed that approximately 60–80 million couples worldwide experience infertility annually, with a notable proportion of 15 to 20 million (25%) representing cases exclusive to India. In accordance with World Health Organization (WHO) data, one out of every four couples in developing nations are confronted with issues of infertility. Infertility is a growing problem that calls for urgent action, especially for the avoidable cases of infertility.[8] Purkayastha et al. elaborates that WHO assessed within the context of India, the occurrence of primary infertility falls within the range of 3.9% to 16.8%.[9] Further, it is estimated by Rahi et al. that in Jammu and Kashmir the prevalence of infertility is 15% with the of higher prevalence of 15.7% in region of Kashmir.[10]
Factors associated with infertility
Age, hormonal imbalances, structural difficulties, male factors, lifestyle variables, medical diseases, environmental factors, and psychological factors are all connected with infertility (Figure 1). Infertility is a complicated issue, with different causes for different people. Some of the factors associated with infertility are listed below.
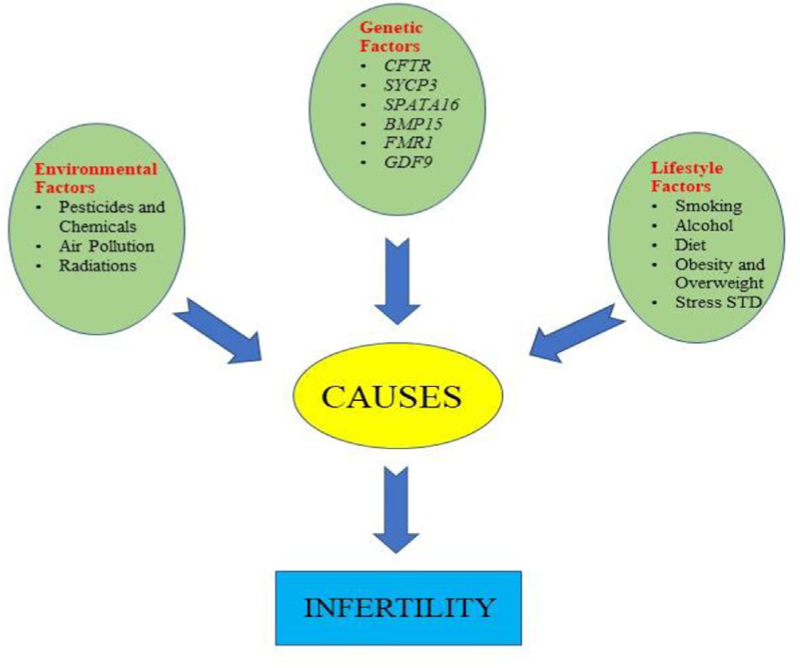
- Shows the co-relation between the genetics, environmental, and lifestyle factors in the progression of infertility.
Genetics
The genetic variables may have an impact on sperm motility, maturation, and production in cases of male infertility. Important genes such as Y chromosomal microdeletions, CFTR, SYCP3, CATSPER1, TEX11, and FSHR have been linked to male infertility. Genetic variables may have an impact on hormone signaling, the development of follicles, and ovarian function in female infertility. Important genes like BMP15, FMR1, ESR1, FSHR, GDF9, and LHCGR are linked to female infertility.
The following genes have been linked to male infertility:
-
CFTR: The cystic fibrosis transmembrane conductance regulator (CFTR) gene results in a protein that controls the movement of chloride ions within cells. Cystic fibrosis, which affects male fertility by blocking the vas deferens or causing aberrant sperm function, can be brought on by mutations in this gene.[11] The quintessential anomaly in the reproductive tract commonly connected with CFTR mutations is solitary congenital bilateral absence of the vas deferens (CBAVD), which is frequently first identified during urological assessments for male infertility. In clinical assessments, a usual finding is the complete bilateral lack of the vas deferens and the lower portion of the epididymis, accompanied by underdevelopment or nonexistence of seminal vesicles.[12]
The absence of vas deferens on both sides strongly indicates the presence of CFTR allelic variations. As per the findings of a meta-analysis carried out by Yu et al. in 2012, it was observed that around 78% of males exhibiting congenital bilateral absence of the vas deferens (CBAVD) were found to possess at least one detectable mutation in CFTR. Further analysis revealed that among the individuals studied, 46% exhibited mutations in both gene copies (biallelic mutations), while 28% displayed a mutation in a single gene copy.[13,14]
SYCP3: During meiosis, the process by which sperm cells are created, the SYCP3 (synaptonemal complex protein 3) gene is involved in the pairing and segregation of chromosomes. Reduced fertility and aberrant sperm production may result from mutations in this gene.[15] Mutations within the SYCP3 gene have been linked to the interruption of meiotic progression in spermatogenesis. This gene's involvement in male infertility had previously been established in mice, where male homozygous null mutants lacking the SYCP3 gene exhibited infertility due to significant apoptotic cell loss during spermatogenesis. Upon the identification of the human SYCP3 gene, it became evident that it serves an analogous role, leading to comparable detrimental effects on human fertility when its function is disturbed.[16] It is noteworthy that, unlike in mice where two mutated alleles are necessary to manifest an infertility phenotype, a solitary heterozygous mutation in the human SYCP3 gene is adequate to hinder spermatogenesis.[17]
-
SPATA16: The SPATA16 (spermatogenesis-associated 16) is expressed in the testes and is involved in the production of sperm. Reduced fertility and poor sperm morphology are two effects of this gene mutation.[18]
In recent research, indications have emerged pointing to the involvement of the SPATA16 gene in the process of sperm production. This gene has been associated with conditions such as arrested spermatogenesis, testicular diseases, globozoospermia, and the occurrence of sperm with an abnormal number of chromosomes (sperm aneuploidy) in humans.[19,20] The SPATA16 protein is found in both the Golgi apparatus and the proacrosomic vesicles. These vesicles are carried to the acrosome in round and elongated spermatids as they undergo spermiogenesis. Research findings align with the idea that SPATA16 plays a vital part in acrosome formation. The most pronounced preservation of the protein occurs within the TPR domain, which experiences changes in cases of globozoospermia.[18,21]
TEX11: In male germ cells, the TEX11 (testis expressed 11) gene is involved in meiosis and DNA repair. By affecting sperm production and quality, alterations in this gene can lead to male infertility.[22]
Given that TEX11 fulfills a crucial function in meiotic recombination and the arrangement of chromosomes, and a deficiency in TEX11 leads to meiotic arrest leading to male infertility, the investigation of TEX11 mutations has attracted interest in uncovering the underlying origins of male infertility, especially among individuals with azoospermia. Research has demonstrated a range of TEX11 mutations in diverse formats, including silent and missense mutations, alterations within intronic regions, frameshift mutations, and even instances of hemizygous deletions. Currently, a total of 46 distinct TEX11 mutations have been recognized, comprising 24 in men with azoospermia and 22 in fertile individuals. Notably, the prevalence of TEX11 mutation in European descent azoospermic males from Germany was only 2.4%, while the frequency was notably higher at 14.5% among azoospermic males from the United States.[23,24]
The following significant genes have been linked to female infertility:
BMP15: Bone morphogenetic protein 15 [BMP15] is a gene that produces a protein that is important in follicular development. Mutations in the BMP15 gene can cause premature ovarian failure, a disorder in which the ovaries stop producing eggs before the age of 40.[25] According to Di Pasquale et al.,[26] an A-G transition at position 704 of the BMP15 gene was the first BMP-15 mutation in women to be linked to hypergonadotropic ovarian failure brought on by ovarian dysgenesis. In the pro region of the BMP-15 proprotein, this mutation causes a non-conserved substitution of Y235C (BMP-15Y235C), which has a dominant-negative effect by changing how the wild-type BMP-15 proprotein is processed.
FMR1: Fragile X Mental Retardation 1 [FMR1] mutations have been linked to early menopause-causing premature ovarian insufficiency.[27] To determine how the CGG repeats in the FMR1 gene affect female infertility, a case–control study was conducted. The high-normal alleles of the FMR1 gene, however, were linked to secondary infertility.[28]
GDF9: The growth differentiation factor 9 [GDF9] gene has been associated with early ovarian failure and other reproductive diseases. GDF9 is involved in the development of the follicles.[29] In female patients with primary and secondary infertility, an expressional investigation of the GDF9 gene was conducted. In both primary and secondary infertile women, the GDF9 gene displayed down-regulation when compared to fertile women.[30]
LHCGR: Luteinizing hormone/chorionic gonadotropin receptor [LHCGR] mutations can result in hypergonadotropic hypogonadism, a disorder where the pituitary gland overproduces luteinizing hormone, which impairs fertility.[31]
Environmental factors causing infertility
Human reproduction exhibits a high sensitivity to environmental fluctuations. There is a significant decrease in human fertility in last 50 years.[7] The vast literature suggests that the physical agents that are present in the environment are spread by human activities that can possibly affect the fertility of human beings. Reproduction is very delicate to the chemicals and air pollutants that are generated by chemical industries and agriculture activities.[32]
Air pollution
In the 21st century, air pollution is the most important environmental concern linked to a wide range of health problems. Therefore, it is crucial to comprehend the correlation between air pollution and its impact on human health.[33] The rapid increase in infertility is equal to the escalating release of harmful emissions, it is apparent that the influence of air pollution on human health will inevitably intensify over the upcoming decade.[34] The pollutants in the air works through several Mechanisms that can affect various human physiological functions such as fertility.[35] Numerous air pollutants like volatile organic compounds (VOCs) exert numerous adverse effects on the human reproductive system.[33] Tobacco smoking is an obvious toxin exposure in the air and it is estimated that smoking more than 10 cigarettes a day can reduced fertility.
Hormonal imbalance
The presence of heavy metals, like zinc, copper, lead, and PAH in vehicle exhaust introduces antiestrogenic, estrogenic, and antiandrogenic effects. Consequently, these actions can disrupt the usual processes of gonadal steroid production and gamete formation, ultimately contributing to infertility. An additional element under recent scrutiny, particulate matter PM2.5, tends to accumulate within reproductive organs by breaching the blood-testis, epithelial, or placental barriers, thus upsetting hormone equilibrium and prompting too.[36,37]
Elevated generation of reactive oxygen species resulting from oxidative stress leads to lipid peroxidation and the subsequent fragmentation of sperm DNA, leading to infertility. Furthermore, changes in sperm DNA, particularly through the formation of DNA adducts, especially involving PAH, lead to alterations in the regulation of gene expression and DNA methylation, which are implicated in male infertility.[38]
Consequently, air pollution has emerged as a prominent factor in the contemporary age, precipitating flawed spermatogenesis, amplified fragmentation of sperm DNA, diminished motility, and anomalous morphological transformations, all contributing to the growing prevalence of male infertility.[39,40]
Pesticides
Exposure to various pesticides may cause harmful effects on various organs of the human body, including the reproductive system. A pesticide is one of the dangerous compounds that can affect the semen count of the exposed worker.[41] And it has been proven that the spouses of agricultural workers who directed work with the use of pesticides and chemicals like DDT (Organochlorine pesticide), became the victim of miscarriages, still births, and spontaneous abortions.[42]
Radiations
In modern societies, people are exposed to numerous radiations in their everyday activities, such as using televisions, mobile phones, computer devices, occupational tools, or even essential medical devices like diagnostic imaging, interventional radiology procedures, and anticancer therapies.[43] Exposure to intense radiations and other harmful substances can cause infertility.[44] The electromagnetic radiations by the rapid technological enhancement impact the human body genetically as well as biologically. The continuous use of mobile phones, laptops, and power lines are the major source of the exposure.[45]
Lifestyle factors causing infertility
The activities and choices we make in our daily lives play a significant role in influencing our health style factors.[7] The following are some of the major lifestyle factors discussed below:
Smoking
Tobacco smoking is considered one of the biggest reasons for infertility, and it has become a trend among the young generation. Smoking is meant to be the biggest reason for male infertility.[46] It has been negatively connected with sperm count, motility, and morphology.[47] Tobacco smoking is also linked with diseases like respiratory diseases, cardiovascular diseases, and the cancers of lung, urinary bladder, kidney, pancreas, etc. For several years, the relationship between tobacco smoking and infertility has been studied.[7] According to a recent meta-analysis, there is a very negative impact of tobacco on human semen because it leads to a lower sperm count and an increased prevalence of morphological abnormalities, encompassing defects in the head, neck, and tail regions of the spermatozoa.[46] Men who smoke had lower acrosin (ACR), a protease located in the sperm acrosome, activity levels, according to recent studies. This decline was linked to a reduction in the acrosome reaction's inducibility. In spermatozoa from smoking males, researchers discovered lower plasma membrane Ca2+-ATPase (ATP2B4) activity together with higher seminal plasma cadmium contents. They proposed that this impact could be to blame for the reduced motility seen in smokers’ spermatozoa.[48] Checkpoint kinase 1 (CHK1), an enzyme crucial for DNA repair and cell cycle regulation, has been shown to express less often in smokers’ spermatozoa. Because the authors found greater sperm DNA fragmentation, they hypothesized that a reduction in CHK1 expression in smokers might lead to impaired sperm DNA repair.[48,49]
Alcohol consumption
Drinking alcohol has its own health and social consequences. Alcohol consumption is associated with various diseases and infertility in both males and females.[50] Numerous clinical and experimental investigations have unequivocally established that alcohol consumption constitutes a risk factor for infertility. It can adversely impact reproductive hormones and the quality of semen.[51] There are several studies, which advised that drinking alcohol and tobacco smoking are the avoidable factors which actually affects the male fertility.[52] And when we talk about unhealthy lifestyle, it comes on the top of the list.[53] The influnce of excess alcohol on hypothalmic on the hypothalamic-pituitary axis is most likely responsible for male reproductive impairment. Ethanol has been shown in models of animals to inhibit GnRH release in the hypothalamus by decreasing the quantity of tiny GTP-binding proteins of the Rab family, which is an essential controller of membrane and protein trafficking.[54,55] An upsurge in oxidative stress at the pituitary level has also been proposed. Alcoholism results in low sperm quality in terms of sperm concentration, motility, and typical characteristics. It also reduces FSH and LH levels, but its impact on T (Testosterone) levels is debatable.[55]
Diet
In both male and female, nutrition plays an important role. A healthy diet in reproductive age of male and female is proved to be a beneficial effect on fertility.[56] The co-relation between the diet and human fertility has been studied from the past decade to get few more clear patterns.[57] Likewise, gynecological diseases cause women infertility, environmental factors, and lifestyle factors like stressful jobs, unhealthy diet, bad nutrition, etc. effect the fertility in both males and females.[58]
Age
Age is an important factor in infertility in both males and females. Because of their pursuit of education and other reasons, most couples delay their child bearing.[59] In both natural and assisted reproduction, age plays an important role. And because of late childbearing many couples face problems like infertility.[60] In last few decades, this aspect has been recognized because of the trend of increased age, in both males and females, achieving in their first pregnancy.[61]
Stressful lifestyle
In all cultures and societies, infertility is a biggest growing complication that has affected almost 80 million people worldwide.[62] The correlation between stress and infertility has been a topic of ongoing debate for many years, and it is not yet clear whether stress causes infertility or infertility causes stress.[63] Stress in any form, whether it is social, physical, or psychological, is the most important part of any society.[59] It is often associated with societal pressure, testing, diagnosis, treatments, failure of treatments, unfulfilled wishes and desires, and even fiscal costs.[59] Infertility is an emotional thing for a couple. And counseling of infertile patients may prove to be helpful throughout their infertility treatment, or it can be a final major help for couples to deal with infertility problem.[64]
Obesity and overweight
Obesity is an escalating health concern among individuals of reproductive age. The excessive fat that affects the body in a negative way causes obesity and overweight.[65] Having a body weight more than 20% of the ideal age is considered obese, and possessing a body mass index ranging from 25 to 29.9 indicates being overweight.[66] Like other health risks, obesity and overweight have also affected the male and female infertility.[59] Vahrati and Smith have found that the most of the women seeking infertility treatments are obese.[65] An abnormal level of reproductive hormones has been found in obese persons. It is a serious disease, which comes along with high cholesterol level, cancer, diabetes, gall bladder disease, heart disease, insomnia, and renal failure.[66]
Sexually transmitted diseases
In both males and females, sexually transmitted diseases can cause permanent damage to the reproductive tract, resulting in infertility.[67] It particularly occurs in the people of aged group 15–50. Prevention from STDs is important, as it leads to acute infections and complications like infertility.[68]
CO-RELATION BETWEEN GENETICS, ENVIRONMENTAL, AND LIFESTYLE FACTORS IN THE PROGRESSION OF INFERTILITY
The movement of barrenness can be impacted by a mix of hereditary, ecological, and way of life factors. Hereditary variables might influence ripeness by causing anomalies in regenerative organs or hormonal uneven characters. Reproductive organs can be harmed, hormonal balance can be disrupted, and DNA damage to eggs and sperm can occur from environmental factors like exposure to toxins and chemicals. The quality and quantity of sperm and eggs, as well as the hormonal balance and overall reproductive health, can all be negatively impacted by lifestyle factors like smoking, alcohol consumption, poor nutrition, and stress. It is difficult to predict how infertility will progress due to the complex interactions between genetic, environmental, and lifestyle factors that can vary from person to person. For instance, while genetic factors may make some people more susceptible to environmental toxins, others may be able to tolerate higher levels of exposure. The movement of barrenness can be impacted by a mix of hereditary, ecological, and way of life factors. Individuals and couples can utilize this information to make informed choices regarding their reproductive health and to pursue suitable medical interventions.
Summary
A mix of genetic, environmental, and lifestyle factors can contribute to infertility. Genetic factors may have an impact on reproductive health, but environmental factors including exposure to pollutants and chemicals, lifestyle factors like smoking, drinking, eating poorly, and stress can also have a big impact. Infertility may also be influenced by other medical issues such obesity, diabetes, and autoimmune diseases. In order to improve reproductive health, understanding the numerous factors that affect fertility can help guide the development of personalized treatment plans and lifestyle changes. Couples who are experiencing infertility may gain from changing their eating patterns, exercise routines, and level of exposure to pollutants in the environment.
Throughout the review, we emphasized that infertility is attributed to a combination of genetics, environmental factors, and lifestyle decisions. Fertility can be affected by genetic disorders such as chromosomal abnormalities and gene mutations, as well as environmental variables and lifestyle behavior. The rising prevalence of infertility necessitates a focus on these variables. We may work towards minimizing the obstacles of infertility and offering improved prospects for people and couples wishing to conceive by recognizing the genetic and environmental components and supporting healthier lifestyles.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Etiology and risk factors associated with infertility. Int J Women's Heal Reprod Sci. 2019;7:346-53.
- [CrossRef] [Google Scholar]
- Definition and causes of infertility. Reprod Biomed Online. 2001;2:41-53.
- [CrossRef] [PubMed] [Google Scholar]
- Investigating marital relationship in infertility: a systematic review of quantitative studies. J Reprod Infertil. 2012;13:71-80.
- [Google Scholar]
- Environment, lifestyle, and female infertility. Reprod Sci. 2021;28:617-38.
- [CrossRef] [PubMed] [Google Scholar]
- Male infertility. Current life style could be responsible for infertility. MMW Fortschr Med. 2000;142:31-3.
- [Google Scholar]
- Environmental factors and male infertility. Spermatozoa Facts and Perspectives 2018:159-71.
- [CrossRef] [Google Scholar]
- Prevalence of primary infertility and its associated risk factors in urban population of central India: a community-based cross-sectional study. Indian J Commun Med. 2019;44:337.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and potential determinants of primary infertility in India: evidence from Indian demographic health survey. Clin Epidemiol Global Health;. 2021;9:162-170.
- [CrossRef] [Google Scholar]
- CFTR mutations and polymorphisms in male infertility. Int J Androl. 2004;27:251-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical genetic testing for male factor infertility: current applications and future directions. Int J Androl. 2014;2:339-50.
- [CrossRef] [PubMed] [Google Scholar]
- CFTR mutations in men with congenital bilateral absence of the vas deferens (CBAVD): a systemic review and meta-analysis. Hum Reprod. 2012;27:25-35.
- [CrossRef] [PubMed] [Google Scholar]
- Genetics of CFTR and male infertility. Transl Androl Urol. 2021;10:1391-1400.
- [CrossRef] [PubMed] [Google Scholar]
- Male infertility and its genetic causes. J Obstet Gynaecol Res. 2015;41:1501-5.
- [CrossRef] [PubMed] [Google Scholar]
- Azoospermia in patients heterozygous for a mutation in SYCP3. Lancet. 2003;362:1714-9.
- [CrossRef] [PubMed] [Google Scholar]
- Disease gene discovery in male infertility: past, present and future. Hum Genet. 2021;140:7-19.
- [CrossRef] [PubMed] [Google Scholar]
- Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am J Hum Genet. 2007;81:813-20.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular analysis of DPY19L2, PICK1 and SPATA16 in Italian unrelated globozoospermic men. Life (Basel). 2021;11:641.
- [CrossRef] [PubMed] [Google Scholar]
- Association of CATSPER1, SPATA16 and TEX11 genes polymorphism with idiopathic azoospermia and oligospermia risk in Iranian population. BMC Med Genomics. 2022;15:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Gene functional research using polyethylenimine‐mediated in vivo gene transfection into mouse spermatogenic cells. Asian J Androl. 2006;8:53-9.
- [CrossRef] [PubMed] [Google Scholar]
- Six polymorphisms in genes involved in DNA double-strand break repair and chromosome synapsis: association with male infertility. Syst Biol Reprod Med. 2015;61:187-93.
- [CrossRef] [PubMed] [Google Scholar]
- TEX 11 is mutated in infertile men with azoospermia and regulates genome‐wide recombination rates in mouse. EMBO Mol Med. 2015;7:1198-210.
- [CrossRef] [PubMed] [Google Scholar]
- X-linked TEX11 mutations, meiotic arrest, and azoospermia in infertile men. N Engl J Med. 2015;372:2097-107.
- [CrossRef] [PubMed] [Google Scholar]
- Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25:279-83.
- [CrossRef] [PubMed] [Google Scholar]
- Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet. 2004;75:106-11.
- [CrossRef] [PubMed] [Google Scholar]
- The FMR1 gene, infertility, and reproductive decision-making: a review. Front Genet. 2014;5:195.
- [CrossRef] [PubMed] [Google Scholar]
- The association of FMR1 gene (CGG) n variation with idiopathic female infertility. Arch Med Sci. 2021;17:1303.
- [CrossRef] [PubMed] [Google Scholar]
- BMP15 and GDF9 gene mutations in premature ovarian failure. J Reprod Infertil. 2017;18:185.
- [Google Scholar]
- Association between gene expression levels of GDF9 and BMP15 and clinicopathological factors in the prognosis of female infertility in northeast Indian populations. Meta Gene. 2021;30:100964.
- [CrossRef] [Google Scholar]
- Genetic evidence of ‘genuine’empty follicle syndrome: a novel effective mutation in the LHCGR gene and review of the literature. Hum Reprod. 2017;32:944-53.
- [CrossRef] [PubMed] [Google Scholar]
- Contribution of environmental factors to the risk of male infertility. Hum Reprod. 2001;16:1768-76.
- [CrossRef] [PubMed] [Google Scholar]
- Air pollution and infertility-a letter to editor. J Environ Treat Tech. 2018;6:72-3.
- [Google Scholar]
- Air pollution and female fertility: a systematic review of literature. Reprod Biol Endocrinol. 2018;16:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Does air pollution play a role in infertility?: a systematic review. Environ Health. 2017;16:1-16.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of PM2. 5 exposure on reproductive system and its mechanisms. Chemosphere. 2021;264:128436.
- [CrossRef] [PubMed] [Google Scholar]
- Does air pollution play a role in infertility?: A systematic review. Environ Health. 2017. 2017;16:1-16.
- [CrossRef] [PubMed] [Google Scholar]
- Concentration of oxygenated polycyclic aromatic hydrocarbons and oxygen free radical formation from urban particulate matter. J Toxicol Environ Health A. 2007;70:1866-9.
- [CrossRef] [PubMed] [Google Scholar]
- Polycyclic aromatic hydrocarbon-DNA adducts in human sperm as a marker of DNA damage and infertility. Mutat Res. 2003;535:155-60.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of environmental factors on human semen quality and male fertility: a narrative review. Ann Hum Biol. 2022;34:1-13.
- [CrossRef] [Google Scholar]
- Occupational exposure to pesticides and consequences on male semen and fertility: a review. Toxicol Lett. 2014;230:146-56.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of oxidative stress on infertility, with emphasis on infertility management strategies. Global J Fertil Res. 2019;4:010-8.
- [CrossRef] [Google Scholar]
- Radiations and female fertility. Reprod Biol Endocrinol. 2018;16:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Radiations and male fertility. Reprod Biol Endocrinol. 2018;16:1-16.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of non-ionizing electromagnetic radiation on male infertility: an assessment of the mechanism and consequences. Int J Radiat Biol. 2020;98:1-24.
- [CrossRef] [PubMed] [Google Scholar]
- Tobacco smoking and semen quality in infertile males: a systematic review and meta-analysis. BMC Public Health. 2019;19:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Lifestyle causes of male infertility. Arab J Urol. 2018;16:10-20.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of risk factors for male infertility on sperm protein composition. Int J Mol Sci. 2021;22:13164.
- [CrossRef] [PubMed] [Google Scholar]
- Potential effect of smoking on semen quality through DNA damage and the downregulation of Chk1 in sperm. Mol Med Rep. 2016;14:753-61.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of alcohol consumption on male infertility. In: Male Infertility. Springer 2014:83-92.
- [CrossRef] [Google Scholar]
- Smoke, alcohol and drug addiction and male fertility. Reprod Biol Endocrinol. 2018;16:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of smoking and alcohol consumptionon reproductive and metabolic indicators in young men in western siberia. Urologiia. 2017;4:62-67.
- [CrossRef] [Google Scholar]
- Impact of smoking and alcohol consumption on oxidative status in male infertility and sperm quality. Indian J Pharm Sci. 2019;81:933-45.
- [CrossRef] [Google Scholar]
- Exposure to ethanol induces oxidative damage in the pituitary gland. Alcohol. 2005;35:91-101.
- [CrossRef] [PubMed] [Google Scholar]
- Addiction and human male fertility: a systematic review and a critical appraisal. Andrology. 2022;10:1073-95.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of diet on fertility and the implications for public health nutrition in the United States. Front Public Health. 2018;6:211.
- [CrossRef] [PubMed] [Google Scholar]
- Diet and fertility: a review. Am J Obstet Gynecol. 2018;218:379-89.
- [CrossRef] [PubMed] [Google Scholar]
- Nutrition and female fertility: an interdependent correlation. Front Endocrinol. 2019;10:346.
- [CrossRef] [PubMed] [Google Scholar]
- Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol. 2013;11:1-15.
- [CrossRef] [PubMed] [Google Scholar]
- Knowledge about the impact of age on fertility: a brief review. Upsala J Med Sci. 2020;125:167-74.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of aging on fertility: similarities and differences between ovaries and testes. Testes and Ovaries: Functional and Clinical Differences and Similarities; 2017. p. :15.
- [CrossRef] [Google Scholar]
- The relationship between stress and infertility. Dialog Clin Neurosci. 2018;20:41.
- [CrossRef] [PubMed] [Google Scholar]
- Emotional aspects of infertility. Fertil Steril. 1982;37:137-45.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of obesity on infertility in women. J Turk Ger Gynecol Assoc. 2015;16:111.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity in relation to Infertility. Res J Pharm Technol. 2018;11:3183-5.
- [CrossRef] [Google Scholar]
- Sexually transmitted diseases and infertility. Sexually Transmitted Dis. 1994;21:S32-7.
- [Google Scholar]
- Epidemiology of sexually transmitted diseases: the global picture. Bull World Health Organ. 1990;68:639.
- [CrossRef] [Google Scholar]







