Translate this page into:
Critical ovarian hyperstimulation syndrome and its management
Address for correspondence: Papa Dasari, Senior Professor, Department of Obstetrics and Gynaecology, JIPMER, Puducherry, India. E-mail: dasaripapa@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dasari P, Rao M. Critical ovarian hyperstimulation syndrome and its management. Fertil Sci Res 2023;10:164-9.
Abstract
Critical ovarian hyperstimulation syndrome during assisted reproductive techniques (ARTs) is a life-threatening condition and is preventable when adequate steps are taken at the right time. A 30-year-old woman, who is married for 10 years, with known polycystic ovarian syndrome (PCOS), type II diabetes mellitus (DM), and hypothyroidism, was transferred to our center in critical condition a day after oocyte retrieval. This is her second intracytoplasmic sperm injection (ICSI) cycle and she was given growth hormone along with antagonist and 23 oocytes were retrieved. In the past, she had more than 12 cycles of ovulation induction, ovarian drilling, and one cycle of ICSI with 3 embryo transfers, which did not result in pregnancy. She had tachycardia, tachypnea, decreased saturation, hypotension, tense ascites, and oliguria. She was resuscitated and the computed tomography pulmonary angiogram (CTPA) showed pulmonary thromboembolism. She was managed in the intensive care unit (ICU) with intravenous (IV) fluids, oxygen, IV heparin, and tablet cabergoline 0.5 mg (Sun Pharma, Bayer House, 2nd Floor, Central Avenue, Hiranandani Estate, Thane (W) 400 607. Maharashtra, India). An abdominal paracentesis was done due to increasing distension and tachypnea and >1700 mL hemorrhagic fluid was drained. The drain was kept for 1 week, and she was started on oral anticoagulants (rivaroxaban 15 mg, Bayer Pharmaceuticals) twice daily after 10 days. She was discharged with advice to continue medication for 12 weeks.
Keywords
Growth hormone
Antagonist
Ovarian hyperstimulation syndrome
Critical
Pulmonary thromboembolism
Hemorrhagic ascites
Pleural effusion
INTRODUCTION
Critical ovarian hyperstimulation syndrome (OHSS) is a life-threatening event, and its occurrence, though rare, needs to be reported. Severe and critical OHSS are rarely encountered in the current era, where antagonist protocols are the norm in almost all centers and mostly all are OHSS-free clinics. In the UK, the occurrence of severe OHSS and critical OHSS is to be reported within 12 hours verbally and in 24 hours with completion of incident form as per Human Fertilisation and Embryology Authority (HFEA) guidelines.[1] Delay in recognition and management of severe OHSS can lead to mortality.[2] The annual incidence of severe OHSS over 17 years in Denmark was reported to vary from 0.9 to 1.2–1.4%, with no overall change over time [1.2% among 186,168 stimulated; in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycles].[3] In this report, we share the diagnosis and management of a case of critical OHSS, who was transferred to our hospital a day after oocyte retrieval.
Case
A 30-year-old woman, Mrs N, who underwent oocyte retrieval a day earlier, was transferred with a provisional diagnosis of OHSS with suspicion of pulmonary thromboembolism. The specialist, who accompanied the patient, gave a history of retrieving 23 oocytes the previous day and a treatment history of administering inj. cetrorelix 1 g subcutaneous (s.c., Manus Aktteva Biopharma, Ahmedabad, Gujarat), tablet cabergoline 2 gm, and inj heparin 5000 IU s.c. The protocol used involved an antagonist with the addition of growth hormone (GH) as adjuvant.
On examination, the patient was drowsy, (GCS E4V5M6) tachypneic, with a respiratory rate of 36/min. Her pulse rate was 120 to 140/min, and her blood pressure (BP) was 90/50 mm Hg. Her SPO2 was 95% with 5 L of oxygen, dropping to 85% in room air. Respiratory system showed decreased breath sounds on auscultation, while the cardiovascular system showed tachycardia and no murmurs. The abdomen was edematous, grossly distended, and tender, and the girth was 117 cm. There was 50 mL of high colored urine in the Urobag. She was admitted to the intensive care unit (ICU) and was resuscitated. During the first hour, 1 L of Ringer lactate was infused. BP came up to 130/100 mm Hg, and the pulse rate was 124/min. Inj. heparin 12,500 IU (Celon Laboratories Ltd, New Delhi, India) was given intravenously.
Abdominal ultrasonography (USG) revealed echogenic fluid. The uterus measured 6.5 × 4.9 cm; endometrial thickness was 4.6 mm; and the left ovary measured 14 × 11 × 13 cm with multiple follicles, and the right ovary measured 8 × 6 × 5 cm. Free fluid was present in the pelvis and hepatorenal pouch. USG thorax showed bilateral pleural effusion, anterior lung sliding, and posterior basal B line. The echocardiogram (ECHO) showed normal left ventricular function, right ventricular dyskinesia, and no pericardial effusion. The ECG showed a heart rate of 110/min, “ST” depression in leads I and II, “T” wave depression in v4 to v6, poor R wave progression, and “Q” waves were present in lead III.
The investigations on transfer (just prior to admission) were as follows: hemoglobin (Hb) 13.8 g%; hematocrit 56.8%, platelets 3.34 lakhs/mm3, white blood cell (WBC) 32,700/mm3; D-dimer was 2.24; prothrombin time (PT)/international normalized ratio (INR) 16/1.2; urea 17, creatinine 0.7; liver function tests (LFTs)—total bilirubin 0.49 mg/dL, alanine aminotransferase (ALT) 78, aspartate aminotransferase (AST) 67; total protein 5.7 g/dL, albumin 3; serum thyroid-stimulating hormone (TSH) was 11 mIU/L.
Emergency computed tomography pulmonary angiogram (CTPA), undertaken after initial stabilization, showed hypodense filling defects in segmental branches of the left lower lobe and lingular segment, suggestive of thrombus, moderate pleural effusion, passive atelectasis of the lower lobe, and atelectatic changes in the lower lobe [Figure 1]. A diagnosis of critical OHSS was made. Inj. heparin was continued at a dose of 5000 IU every 6 hours intravenously. She was receiving 8 L of oxygen via a mask. Abdominal girth increased to 118 cm over 6 hours, and tachypnea persisted and hence USG-guided abdominal drain was kept in the right flank. More than a liter of bloody fluid was drained, and the patient felt immediate relief from respiratory embarrassment, which she expressed.
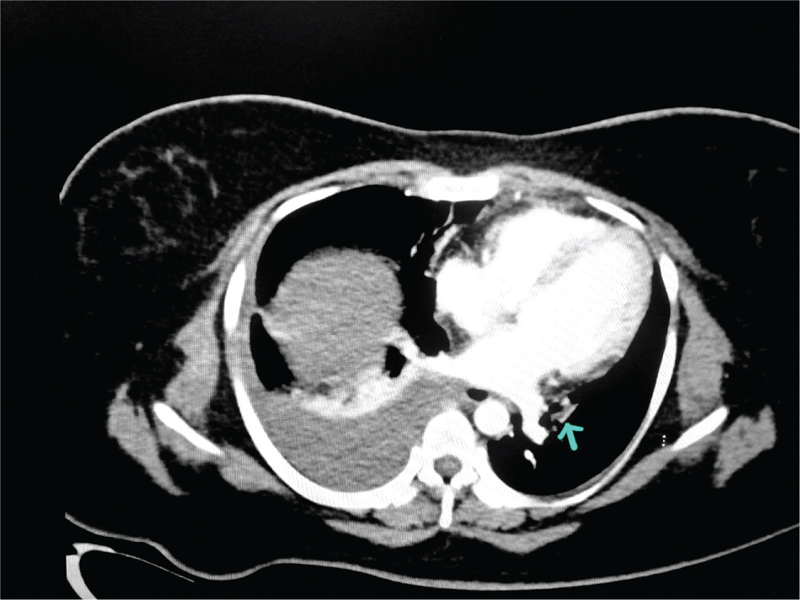
- Computed tomography pulmonary angiogram (CTPA) at admission. Hypodense filling defects in segmental branches of left lower lobe and lingular segment segments of left upper lobe s/o pulmonary thromboembolism. Main/right/left pulmonary artery shows normal opacification. Right moderate pleural effusion with passive atelectasis of lower lobe and posterior segment of upper lobe. Arrow points to pulmonary thrombus.
Her menstrual history was that she has been experiencing irregular cycles for the past 10 years and gets withdrawal bleeding, only when she takes hormones. Her medical history revealed that she is hypothyroid and consuming 125 µg of levothyroxine (Eltroxin) daily. She has been married for 10 years, and her husband is a 40 years old car mechanic. His semen analysis reported teratozoospermia, and he was clinically diagnosed to be having varicocele and he did not receive any treatment. She was diagnosed as polycystic ovarian syndrome (PCOS) and underwent hysterosalphingogram (HSG), hysterolaparoscopy, and ovarian drilling in 2015. From 2015 to 2018, she underwent multiple cycles of ovulation (>12 cycles) induction with timed coitus. In 2018, she underwent ICSI at another assisted reproductive technique (ART) center and had embryo transfer twice during which three good-quality embryos were transferred but not implanted. In 2019, she attempted a cycle of intrauterine insemination (IUI). She was diagnosed as type II diabetes mellitus (DM) a month prior to this cycle of stimulation (March 2023) and was on tablet metformin 500 mg twice daily. Her anti-mullerian hormone (AMH) level was measured at 5.4 ng/mL; follicle-stimulating hormone (FSH) at 3.94 IU/L; luteinizing hormone (LH) at 4.52 IU/L; prolactin at 14 ng/L; and TSH at 11 IU/L.
She was treated with intravenous (IV) heparin, broad-spectrum antibiotics, IV albumin, and IV fluids as per central venous pressure (CVP). Her oxygen requirement gradually decreased to 2 L/min in 4 days, and she could maintain an SPO2 of 96% on room air after 6 days and she was shifted to High Dependency Unit (HDU). The drain fluid got cleared to serous after 7 days and decreased to < 300 mL/day after which it was removed. A repeat CTPA on day 9 of admission showed resolution of pleural effusion and persistent thrombus in segmental branches of the left pulmonary artery [Figure 2]. Her investigations and progress are presented in Tables 1 and 2, respectively. She was started on oral anticoagulation, rivaroxaban 15 mg twice daily from day 10 as per pulmonologists advice. She was discharged on day 14 with advice to continue oral anticoagulants for 3 months and to return for follow-up after 3 weeks.
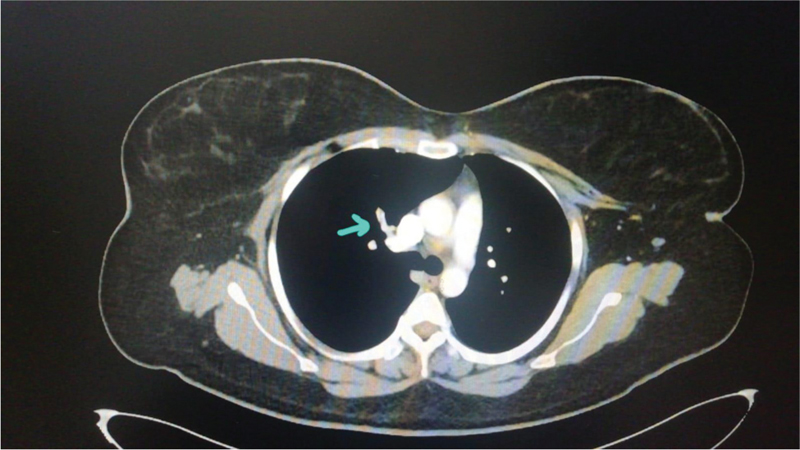
- Computed tomography pulmonary angiogram (CTPA) day 9. Nonenhancing partial filling defect seen in segmental branches of right upper lobe pulmonary artery—s/o pulmonary thromboembolism. Suspicious tiny filling defect is also seen in segmental branch of left upper lobe pulmonary artery to apicoposterior segment. Arrow points to pulmonary thrombus.
| Test Results | Date (Day 1) | Date (Day 2) | Date (Day 3) | Date (Day 4) | Date (Day 5) | Date (Day 8) | Date (Day 12) |
|---|---|---|---|---|---|---|---|
| Hemogram | 20-03-23 | 21-03-23 | 22-03-23 | 23-03-23 | 24-3-23 | 27-03-23 | 31-03-23 |
| Hb (g/dL) | 13.8 | 8.9 | 8.9 | 7.7 | 9.2 | 8.5 | 9.1 |
| PCV (%) | 53.8 | 27 | 27.6 | 25.2 | 30.1 | 26.4 | 28 |
| WBC (/μL) | 32,700 | 12,300 | 11,890 | 7690 | 7740 | 9.490 | 9290 |
| Platelets (/μL) | 3.3 L | 1.38 | 1.69 | 1.42 | 1.76 | 2.07 | 2.65 |
| Liver function tests | |||||||
| Total bilirubin/direct bilirubin (mg/dL) | 0.49/0.23 | 0.35/0.14 | 0.32/0.13 | 0.24/0.08 | 0.4/0.1 | 0.36/0.1 | 0.4/0.1 |
| Serum total protein/albumin (g/dL) | 5.78/3.03 | 4.6/2.8 | 4.4/2.7 | 3.02/1.79 | 5.3/3.4 | 5.4/3.04 | 6.5/3.23 |
| AST/ALT (IU/L) | 78/67 | 56/45 | 42/32 | 52/30 | 23/24 | 41/56 | 23/24 |
| Renal function tests | |||||||
| Urea (mg/dL) | 20 | 13 | 13 | 11 | 11 | 10 | 10 |
| Creatinine (mg/dL) | 1.01 | 0.62 | 0.48 | 0.25 | 0.54 | 0.6 | 0.63 |
| Serum electrolytes | |||||||
| Na+ (mEq/L) | 133 | 131 | 134 | 143 | 135 | 137 | 137 |
| K+ (mEq/L) | 4.5 | 4.2 | 3.75 | 3.4 | 3.4 | 3.9 | 3.95 |
|
Serum estradiol (pg/mL) Special investigations |
22,230 | - | - | - | - | - | 65 |
| PT-INR | 16/1.2 | 16/1.4 | 12.3/1.08 | - | 9.5/0.84 | 10/0.88 | 10/0.88 |
| D-dimer | 2.24 | - | 0.406 | - | 0.406 | - | - |
| aPTT- | - | 54.3 | 20.6 | - | 25.9 | 26.2 | 26.2 |
| Arterial blood gas analysis (ABG) 20-3-2023 | Echocardiography 20-3-2023 | ||||||
| pH | 7.34 | 7.4 | |||||
| HCO3 | 14.6 | 21.5 | LV function normal | ||||
| Pco2 | 27 | 32.6 | No RWMA | ||||
| Po2 | 110 | 82.8 | B/L pleural effusion | ||||
| So2 | 97.8 | 96.61 | |||||
| TAS 20-3-2023. | TAS 31-3-2023 | ||||||
| Uterus measuring 6.5×4.9 cm | Uterus 6.4 × 5 cm | ||||||
| ET 4.6 mm | ET 4.5 mm | ||||||
| Left ovary 14.7 ×11.6× 12.6 cm | Left ovary 5.6 × 2.9 cm | ||||||
| Right ovary 8.1 × 6.03 × 6.5 cm | Right ovary 4 × 3.8 cm | ||||||
| Free fluid seen in hepatorenal pouch TAS 31-3-2023 | Minimal free fluid in POD | ||||||
ALT = alanine transaminase, aPTT = activated partial thromboplastin time, AST = aspartate aminotransferase, ET = Endometrial thickness, Hb = hemoglobin, INR = international normalized ratio, PCV = packed cell volume, POD = Pouch of Douglas, PT = prothrombin time, RWMA = Regional Wall Motion abnormalit, TAS = Trans abdominal Sonography, WBC = white blood cell.
| Parameter | Day 1 | Day 2 | Day 3 | Day 4 |
|---|---|---|---|---|
| GC | Conscious, oriented, breathless and anxious | Fair | Fair | good |
| Pulse | 136/min | 88 | 81 | 75 |
| BP | 140/80 | 120/80 | 118/60 | 105/60 |
| RR | 24/min | 25 | 20 | 20 |
| RS | B/L crepitations | B/L crepitations | Clear | Clear |
| CVS | S1, S2 heard | S1, S2 heard | S1, S2 heard | S1, S2 heard |
| SpO2 | 99% on 4 L nasal prongs | 99% on 4 L nasal prongs | 99% on 4 L of nasal prongs | 99% on 4 L of nasal prongs |
| Abdominal girth | 116 cm | 116 cm | 114 cm | 113 cm |
| Management O2 (L/min) | 4 L by mask | 4 L by nasal prongs | 4 L by nasal prongs | 4 L by nasal prongs |
| IV fluids | 2 pints RL rush8 pints RL + NS +5% D (CVP guided) | 7 pints RL + NS (CVP guided) | 6 pints RL + NS (CVP guided) | 6 pints RL + NS (CVP guided) |
| Heparin | • Inj. UFH 12,500 U STAT followed by 5000U | • Inj. 5000 U 6 hourly | • Inj. 5000 U 6 hourly | • Inj. 5000 U 6 hourly |
| 6 hourly | ||||
| Other drugs | • Inj. cetrolix 0.25 mg i.m. OD | • Inj. cetrolix 0.25 mg i.m. OD | • Inj. cetrolix 0.25mg i.m. OD | • Inj. cetrolix 0.25mg i.m. OD |
| • Inj. ceftriaxone 1 g i.v. q12 hourly | • Inj. ceftriaxone 1 g i.v. q12 | • Inj. ceftriaxone 1 g i.v. q12 | • Inj. ceftriaxone 1 g i.v. q12 | |
| hourly | hourly | hourly | ||
| • Inj. metronidazole 500 i.v. q 8 hourly | • Inj. metronidazole 500 i.v. q 8 | • Inj. metronidazole 500 i.v. q 8 | • Inj. metronidazole 500 i.v. q 8 | |
| hourly | hourly | hourly | ||
| • Inj. hydrocortisone 100mg OD | • Inj. hydrocortisone 100mg OD | • Inj. hydrocortisone 100 mg OD | • Inj. albumin 100mL in NS | |
| • Inj. albumin 100mL in NS | • Inj. albumin 100mL in N.S | • Inj. albumin 100mL in NS | • Tab cabergoline 0.5 mg BD | |
| • Tab Cabergoline 0.5 mg BD | • Tab cabergoline 0.5 mg BD | • Tab cabergoline 0.5 mg BD | ||
| Paracentesis/drain | 1785 mL | 800 mL | 675 mL | 650 mL |
| output Input/output | 5550/795 mL | 4760/2380 mL | 3730/2055 mL | 4700/2380 mL |
BP = blood pressure, CVP = central venous pressure, CVS = Cardiovascular system, GC = General condition, IV = intravenous, NS = Normal Saline, RL = Ringer Lactate, RR = Respiratory Rate, RS = Respiratory System, UFH = Unfractionated Heparin.
DISCUSSION
Ovarian hyperstimulation syndrome develops due to an increase in vascular endothelial growth factor (VEGF) following human chorionic gonadotropin (HCG) administration in a stimulated cycle. It is also reported in spontaneous pregnancies occasionally. VEGF increases the vascular permeability and leads to fluid accumulation and the inflammatory mediators cause thrombosis. The severity of OHSS is classified as mild, moderate, severe, and critical, as published in Green-top guidelines.[4] The features of critical OHSS include tense ascites or hydrothorax, a hematocrit >55%, WBC count >25,000/μL, oliguria or anuria, thromboembolism, and adult respiratory distress syndrome (ARDS). The current patient had almost all these clinical features.
The management of critical OHSS includes the prevention of dehydration and supportive treatment, correction of electrolyte imbalance, and maintenance of acid–base balance. Cabergoline and an antagonist is to be administered for 5 to 7 days. The indications for paracentesis include increasing abdominal distension, persistent pain, shortness of breath, tachycardia, hypotension, hematocrit >45%, and oliguria.[4] This patient had shortness of breath and increasing abdominal girth and hence to avoid repeated paracentesis, an abdominal drain was kept. A case of critical OHSS was reported by Sharma[5] where the woman has presented with acute renal failure, ascites, and pulmonary edema. Serum estradiol levels were >11,000 pg/mL, and she sustained cardiac arrest during the course of management, from which she was revived and required ventilatory support for 3 weeks along with dialysis. She finally recovered after 8 weeks after treatment for severe sepsis
Thromboembolism in OHSS can occur at any part of the body, but it is most commonly reported in the lungs, brain, and heart. It can be both arterial and venous, and the clinical features vary accordingly. A case of right atrial thrombus with pulmonary thromboembolism is recently reported by Sayyadi et al.[6] The case fulfilled all the criteria of critical OHSS with severe pleural effusion of more than a liter, which was subsequently drained. She recovered in 2 weeks’ time and received 80 mg of enoxaparin (Emcure Pharmaceuticals, Pune (Bhosari, Hinjawadi) and Ahmedabad) subcutaneously every 12 hours. A case of pulmonary embolism of left pulmonary artery with intraperitoneal bleeding while on prophylaxis with low molecular weight heparin (LMWH) for OHSS was on record where therapeutic doses of LMWH was given and the intraperitoneal bleeding was managed conservatively.[7] The duration of anticoagulation varies and needs to be individualized based on the risk factors and every attempt to be made to rule out thrombophilias.[4]
There are multiple factors leading to OHSS, including the iatrogenic factors which may be preventable. Patient factors include PCOS, young age, high AMH levels, high gonadotropin dosage, higher number of days of stimulation, HCG trigger despite high estradiol levels, etc. The incidence of moderate OHSS is reported in 3 to 6%, and the severe form occurs in 0.1 to 3% of all cycles, and it can be as high as 20% in women with high risk factors.[8]
The incidence of OHSS was significantly lower with the antagonist protocol compared to the long agonist protocol (5.1 versus 8.9%) reported after a randomized controlled trial (RCT).[9] Based on AMH as a tool, individualized dosing reduced the occurrence of mild and moderate OHSS and subsequently the costs for management of these OHSS in a multicenter trial.[10] When growth hormone is used in the antagonist protocol, there is less requirement for the dose and duration of stimulation, and it significantly increased the mean E2 levels on the day of trigger, even in poor responders. There is no added benefit in terms of clinical pregnancy and live birth rates.[11] The patient in the current study was not a poor responder; her age is 30 years and her AMH was 5.4 ng/mL. She had implantation failure in her previous IVF cycle. However, we could not get the details of gonadotropin dosage. It is to be noted that growth hormone as an adjuvant increases the risk of severe and critical OHSS without increasing pregnancy rates. When controlled ovarian hyperstimulation (COH) for ART is undertaken, stratification into low and high responders is necessary to choose a protocol and the European Society of Human Reproduction and Embryology (ESHRE) guidelines to be followed to prevent OHSS.[12] The preventive strategy in this woman should have been either coasting or administering an agonist trigger. An important part of OHSS prevention is prediction and subsequently selecting the right protocol. The risk of moderate to severe OHSS was estimated to be 2.2% with antral follicle count (AFC) of ≤24 and 8.6% with AFC of ≥24 and this increases further to 11% in women with PCOS.[13]
CONCLUSION
Critical OHSS is a life-threatening condition, and every effort should be made to prevent OHSS. Management should take place in tertiary care centers with facilities for imaging, emergency ventilation, and correction of electrolyte and acid–base balance. Paracentesis helps in decreasing respiratory embarrassment and improving the cardiovascular function. Individualized controlled ovarian stimulation is to be practiced to prevent the development of OHSS based on risk factors of the patient. Coasting/step down protocol, when adjuvants are used, can help to prevent unexpected OHSS in some women.
Declaration of patient informed consent for case reports
The authors declare that the patient consented for publication of the case in an anonymous way. She last attended our OPD on 7-8-23.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Sudden death due to severe ovarian hyperstimulation syndrome: an autopsy-centric case report. Am J Forensic Med Pathol. 2021;42:88-91.
- [CrossRef] [PubMed] [Google Scholar]
- Annual incidence of severe ovarian hyperstimulation syndrome. Dan Med J. 2021;68:A12190738.
- [Google Scholar]
- Management of ovarian hyperstimulation syndrome. Available from https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/the-management-of-ovarian-hyperstimulation-syndrome-green-top-guideline-no-5/ (accessed )
- [Google Scholar]
- Critical ovarian hyperstimulation syndrome and management. J Obstet Gynaecol India. 2016;66:131-3.
- [CrossRef] [PubMed] [Google Scholar]
- Right atrial thrombus and pulmonary thromboembolism related to ovarian hyperstimulation syndrome: a case report and literature review. Clin Case Rep. 2023;11:e7018.
- [CrossRef] [PubMed] [Google Scholar]
- Pulmonary embolism and intraperitoneal bleeding in a patient with severe ovarian hyperstimulation syndrome (OHSS): A management dilemma. Middle East Fertil Soc J. 2018;23:158-60.
- [CrossRef] [Google Scholar]
- Ovarian hyperstimulation syndrome: steps to maximize success and minimize effect for assisted reproductive outcome. Fertil Steril. 2010;94:173-8.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod. 2016;31:1253-64.
- [CrossRef] [PubMed] [Google Scholar]
- OPTIMIST Study Group. Individualized FSH dosing based on ovarian reserve testing in women starting IVF/ICSI: a multicentre trial and cost-effectiveness analysis. Hum Reprod. 2017;32:2485-95.
- [Google Scholar]
- Does the addition of growth hormone to the in vitro fertilization/intracytoplasmic sperm injection antagonist protocol improve outcomes in poor responders? A randomized, controlled trial. Fertil Steril. 2016;105:697-702.
- [CrossRef] [PubMed] [Google Scholar]
- Guideline on ovarian stimulation for IVF/ICSI. 2019. Available from https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Ovarian-Stimulation-in-IVF-ICSI (accessed )
- [Google Scholar]
- Prediction of in vitro fertilization outcome at different antral follicle count thresholds in a prospective cohort of 1,012 women. Fertil Steril. 2012;98:657-63.
- [CrossRef] [PubMed] [Google Scholar]







