Translate this page into:
Dose vitamin D supplementation affect reproductive outcomes in patients with polycystic ovary syndrome?
Address for correspondence: Ayman Shehata Dawood, Department of Obstetrics and Gynecology, Tanta University, Tanta 31111, Egypt. E-mail: ayman.dawood@med.tanta.edu.eg
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
To evaluate the effect of vitamin D supplementation on ovulation and pregnancy rates in patients with polycystic ovary syndrome (PCOS).
Material and Methods:
This multicenter randomized controlled clinical trial was performed in the infertility clinics of Tanta University Hospital and Benha University Hospital. One hundred and sixty patients were recruited and allocated into two groups; group 1 served as a control group and group 2 (study group) where patient received vitamin D supplementation. Induction of ovulation with clomiphene citrate.
Results:
No significant differences were seen in in demographic data: basal hormonal levels, mature follicle size, serum progesterone levels, or pregnancy rate between both groups. Insulin resistance was greater in the control group and resumption of the cycle was higher in the study group.
Conclusion:
Vitamin D supplementation as an adjuvant to induction of ovulation in women with PCOS resulted in enhancement of ovulation and pregnancy rates.
Keywords
Induction of ovulation
infertility
PCOS
pregnancy rate
vitamin D
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a prevalent female endocrine condition that affects between 6% and 12% of women of reproductive age worldwide.[1] It manifests itself through monthly irregularity, hyperandrogenism, anovulatory infertility, obesity, and also metabolic abnormalities such as type 2 diabetes, insulin resistance, and dyslipidemia.[2] These complaints can have a major influence on patients’ health and their life quality. Nevertheless, owing to the disease’s intricacy, the pathophysiology is unknown, and an appropriate therapeutic regimen has not been found.[3] It is typical for people with PCOS to have vitamin D deficiency (VDD), which may be linked to endocrine and metabolic disorders.[4,5,6,7]
Bone mineralization and calcium phosphate control are two of the functions of vitamin D.[8] Over 30 tissues, involving the liver, immune cells, pancreas, brain, and ovaries, have vitamin D receptors that operate as transcription factors for 229 genes.[9] The body obtains vitamin D from vulnerability to the sunshine. Vitamin D is necessary for many functions in the body including immune system, muscle functions, cardiovascular functions, brain development, fertility, and has an anticancer effect.[10]
Vitamin D affects fertility by many mechanisms. Its receptors were detected in the placenta, ovary, and endometrium suggesting that vitamin D has an important role in fertility and conception. Some studies found that deficiency of vitamin D leads to poor response to ovarian stimulation as well as little success with in vito fertilization (IVF). Some studies found that the higher the concentrations of vitamin D, the higher pregnancy success rates. When it comes to PCOS, several studies have established a correlation between lowered vitamin D3 levels and an elevated insulin resistance risk.[5] Vitamin D is accumulated in adipose tissues in obese individuals, rendering it inaccessible for use by the body. Therefore, obese individuals are predicted to have reduced serum vitamin D levels.[11]
PATIENTS AND METHODS
Study design and settings: This multicenter randomized controlled clinical trial was conducted in the infertility clinics of Tanta University Hospital and Benha University Hospital during the period between April 2019 and March 2021.
Patients: One hundred and sixty patients were recruited according to inclusion and exclusion criteria.
The inclusion criteria were: (a) PCOS was diagnosed by modified Rotterdam criteria,[2] which required at least two of these three criteria to make the diagnosis; oligo/anovulation, clinical, or biochemical hyperandrogenism and polycystic ovarian morphology; (b) infertile patients; (c) age between 20 and 35 years; (d) BMI ≤ 30 kg/m2; and (e) vitamin D deficient participants. Exclusion criteria were: (a) pregnant or nursing, (b) kidney stones, (c) current vitamin D use, (d) insulin sensitizing drug use within 3 months, (e) non-PCOS patients, (f) obese patients BMI > 30 kg/m2, and (g) previous ovarian surgery or oophorectomy to one ovary.
Sample size calculation
An independent case-control study with one control for each case was planned. A previous study found that 40% of women in control groups ovulated. Eighty experimental subjects and 80 control subjects were required to reject the null hypothesis that the error rates for both groups are equivalent with a probability (power) of 0.8, if the true ovulation rate for vitamin D sufferers is 0.62. This assay of the null hypothesis has a 0.05 chance of producing a type I error.
Randomization, allocation, and concealment
To produce a random allocation sequence, a computer-generated list was utilized in conjunction with MedCalc software (Medcalc software Version 13.2, Ostend Belgium), version 13.2.2, to allocate each participant’s number to one of the study groups.
Participants were allocated randomly to either group 1 (control group) or group 2 (vitamin D supplementation group) utilizing a computer-generated random number generator.
The assignment was accomplished by the use of sequentially numbered, otherwise identical, sealed envelopes (SNOSE), each of which contained a 2-inch by 2-inch piece of paper with a printed code denoting the assigned group. These documents were placed inside an envelope along with a folded sheet of aluminum foil. Every effort was made to ensure that the intervention and control envelopes were identical in size and weight. Envelopes were selected to be opaque and lined with carbon paper on the inside and were unsealed sequentially only after the subject’s tracking information was written on the envelope, so that the carbon paper functioned as an audit trail.
Interventions
Vitamin D supplementation: oral cholecalciferol (VEDI-PEX 2000 IU tables; PEX Pharma, Egypt). According to the Endocrine Society guidelines, 6000 IU of vitamin D3 were given daily to patients with VDD for 8 weeks to achieve a serum level of 25(OH)D above 30 ng/mL, followed by a maintenance dose of 2000 IU daily for 6 months.
Induction of ovulation with clomiphene citrate: clomiphene citrate’s 50 mg tablets (Tecnovula 50 mg; Techno, Egypt). Regimen: 50 mg daily starting dose of clomiphene citrate was given for 5 days initiating on day 2 of spontaneous or progestin-triggered menstrual bleeding for maximum six cycles with 50 mg dose-increments in subsequent cycles in individuals who unsuccessful to ovulate, up to a daily dosage cap of 150 mg (The Thessaloniki ESHRE/ASRM-Sponsored PCOSConsensus Workshop Group, 2007).[12] Folliculometric follow-up was done every other day starting at the 10th day of the induction cycle. When at minimum one follicle reaches 18 mm, ovulation is stimulated by human chorionic gonadotropin’s 10,000 IU (Choriomon; IBSA, Lugano, Switzerland) intramuscularly with timed intercourse 24 to 36 hours thereafter.
Patients allocated to control group received starch tablets as a placebo in similar manner, dosage, and duration to patients in study group.
Study outcomes: The primary outcome was ovulation detected by transvaginal ultrasound and mid-luteal serum progesterone level (>10 ng/mL).
Secondary outcomes were reversal of insulin resistance as measured by fasting glucose/insulin ratio (ratio becomes <4.5), resumption of regular cycles, and occurrence of pregnancy.
Ethical issues and trial registration
This study was approved by the ethical committee of Tanta University and was given the unique approval code: 35162. The study was also registered on clincaltrials.gov and had the unique ID: NCT03898934 and is accessible on the following link:
Statistical methods
For normally distributed data, mean and standard deviation were used while irregular data were presented as median (25th–75th percentiles). To compare continuous data, either t-test and Mann–Whitney test were used in our study. In order to contrast categorical data presented as frequencies and percentages, the chi-square or Fisher exact test was applied. All analyses were conducted using Stata 16.1 (Stata Corp, College Station, TX, USA), and a P-value < 0.05 was being statistically substantial.
RESULTS
Initial enrollment included 185 patients. Fifteen cases were excluded either due to nonfulfillment of inclusion criteria (n = 9) or declined to participate (n = 6). The flow of cases throughout the study was presented in Figures 1 and 2.
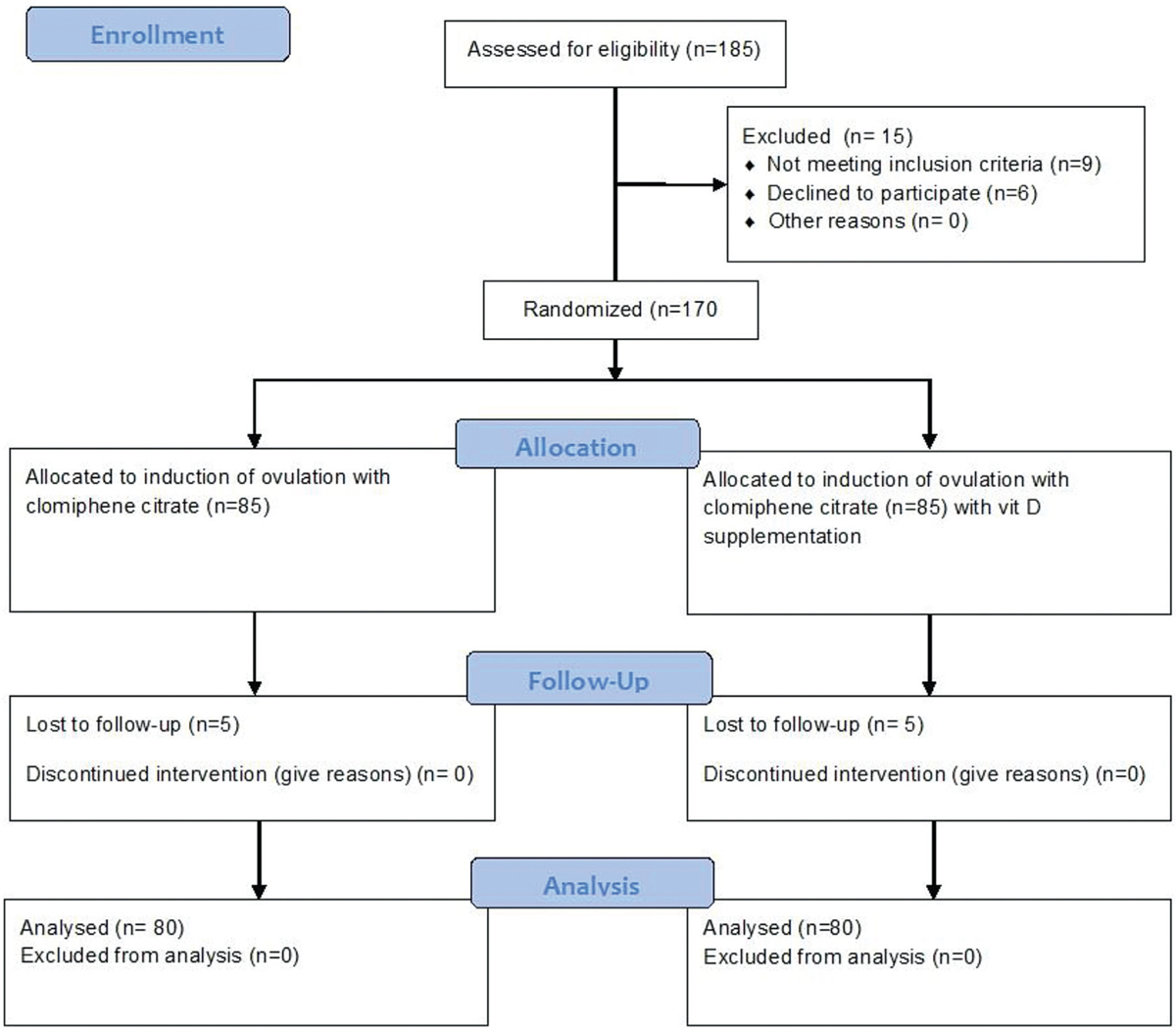
- CONSORT flow chart of cases throughout the study.
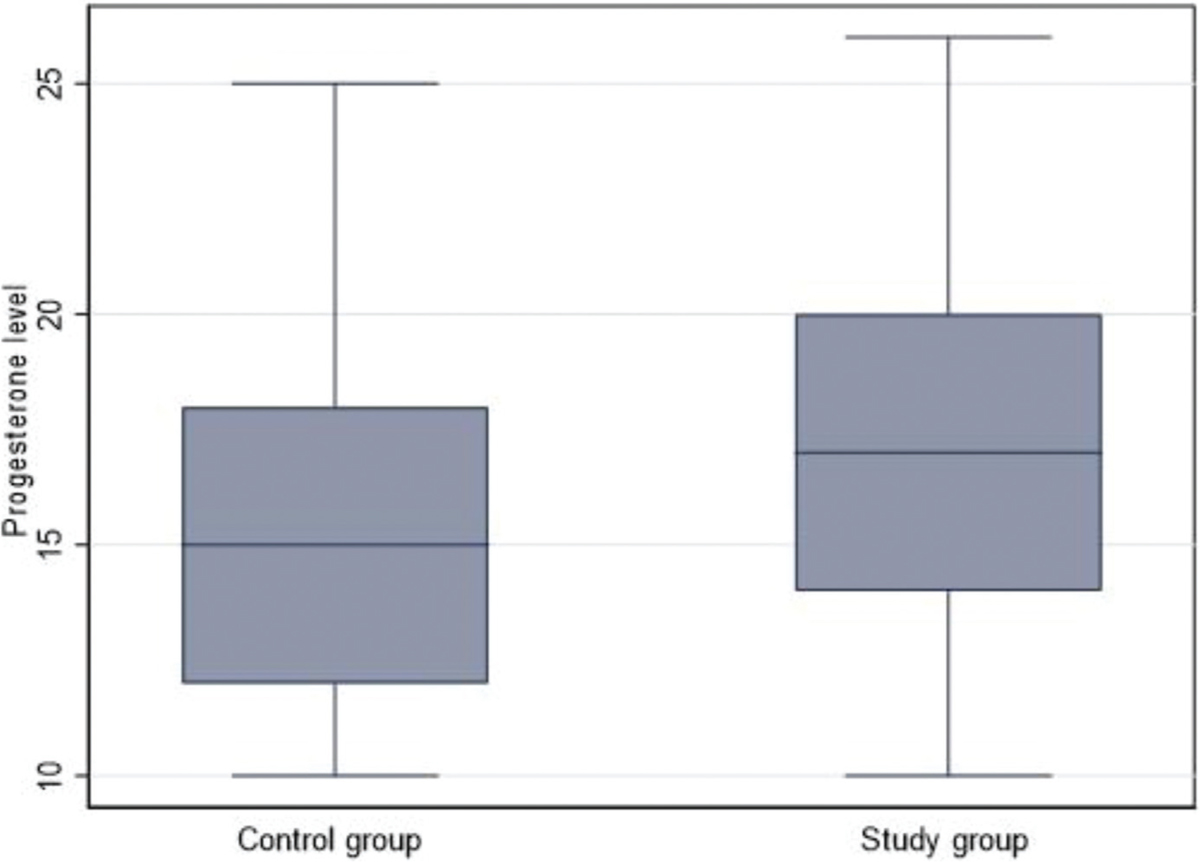
- Box plot of the progesterone level in the control and study group.
Baseline data
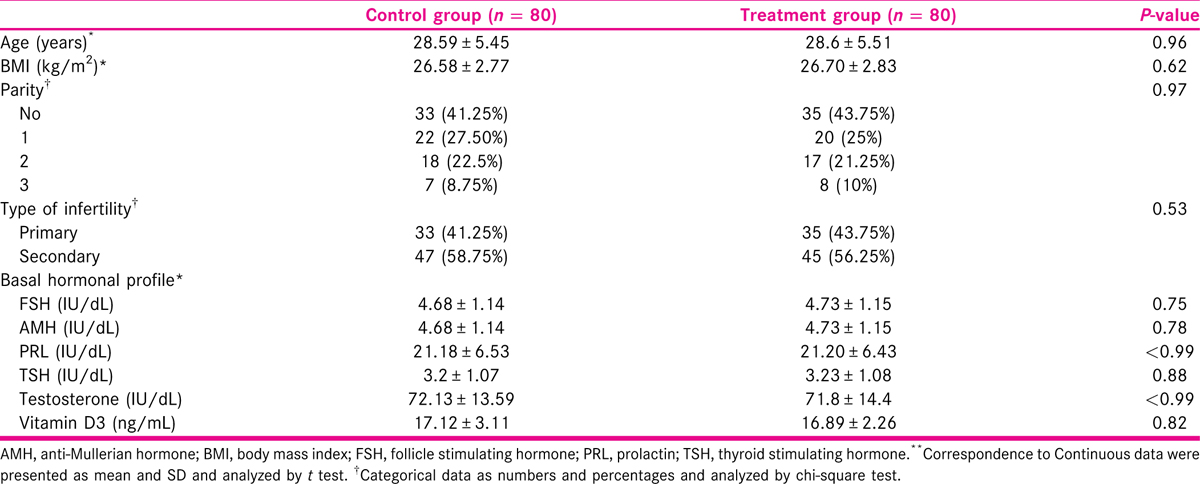
There were no significant differences in demographic data, and hormonal levels between both groups [Table 1].
Clomiphene citrate dose and number of cycles were lower in the study group; however, the number of mature follicles was greater. No significant differences were seen in mature follicle size or serum progesterone levels between the two groups, but the study group’s levels were much greater [Figure 1]. There were no variations in the pregnancy rate between groups. Insulin resistance was greater in the control group and resumption of the cycle was higher in the study group (P ˂ 0.001, for both) [Table 2].
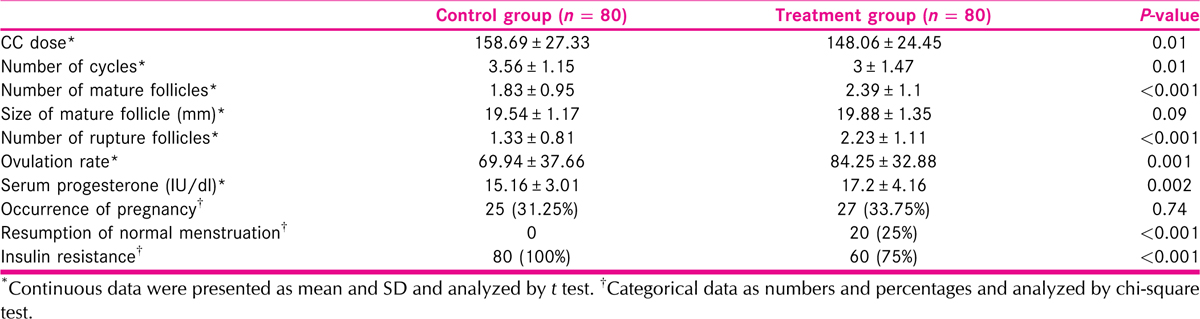
Ovulation was significantly increased in treatment group than control group (p=0.001). On the same side serum progesterone was significantly increased in treatment than in control group as shown in Figure 2.
Factors affecting progesterone level
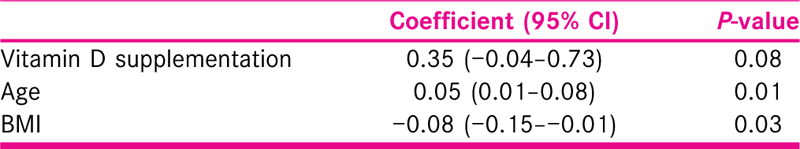
Vitamin D3 supplementations and Prolactin were associated with higher serum progesterone [Table 3].

Factors affecting the size of the mature follicles
The higher size of the mature follicle is significant with increased age and lower BMI. Treatment increased the size of the follicle, but it did not reach a significant level [Table 4].
DISCUSSION
PCOS affects 6% to 12% of reproductive age women over the world. Resistance, loss, and ovarian hyperstimulation syndrome (OHSS) are among the many issues that can arise during the tough induction process. Therefore, we need adjuvant therapy to avoid these complications and improve pregnancy rate. One of the adjuvant therapies is vitamin D.[9,10]
Jamilian et al.[13] conducted a study to assess the effect of vitamin D supplementation on the metabolic profile in women with PCOS. They randomly allocated 90 participants into three groups. Each group included 30 patients where the first group received vitamin D 4000 IU/day and the second group received 1000 IU/day for 12 weeks, while the third group received placebo. They found that high dose of vitamin D (4000 IU) led to a great reduction of total testosterone, inflammatory biomarkers (high sensitivity C-Reactive Protein (hs-CRP) levels), and antioxidant levels in insulin-resistant women with PCOS if compared to those who received 1000 IU/day of vitamin D or placebo.[13]
In a study with Trummer et al.[14], 180 women with PCOS and 25-hydroxyvitamin D concentrations < 75 nmol/L were randomized into 2:1 ratio to receive either vitamin D or placebo by a computer-generated randomization. They aimed to study the effects of vitamin D supplementation on metabolic and endocrine parameters in PCOS. They found no significant effect on metabolic and endocrine parameters in PCOS with the exception of a reduced plasma glucose.[14] Similar results were obtained by Łagowska et al.[15] in their systematic review.
Another study demonstrated that vitamin D supplementation (4000 IU/day) for 12 weeks to PCOS women resulted in significant decreases in serum insulin, homeostatic model assessment for insulin resistance (HOMA-IR), serum total testosterone levels, free androgen index (FAI), and hirsutism, and a significant increase in sex hormone binding globulin (SHBG).[16]
Akl et al.[17] conducted a randomized controlled study to evaluate the effect of vitamin D supplementation on ovulation and metabolic profiles in patients with PCOS in Shams University Maternity Hospital. They included 300 women in their study after assessing their levels of vitamin D. They allocated patients into vitamin D deficient group and vitamin D normal group and control group. They found that vitamin D supplementation improved ovulation rates and metabolic profiles with restoration of menstrual patterns in the group of VDD.[17]
In the present study, we attempted to replenish vitamin D3 level in patients with PCOS through oral vitamin D3 administration. We evaluated ovulation rate, pregnancy rate, serum progesterone, resumption of normal menstruation, and insulin resistance in these patients to those who did not obtain vitamin D3. This was in accordance with the study of Al-Bayyari et al.[18] who recommended supplementation of vitamin D3 to obese patients with PCOS to maintain low levels of vitamin D3 in that category of patients.
In our study, clomiphene citrate (CC) dose and number of cycles were lower in the study group; however, the number of mature follicles was higher. No significant differences were seen in mature follicle size or serum progesterone levels between the two groups, but the study group’s levels were much greater [Figure 1]. There were no variations in the pregnancy rate between groups. Insulin resistance was greater in the control group and resumption of the cycle was higher in the study group.
Numerous publications have proposed a link between VDD and metabolic malfunctions in women with PCOS, notably insulin resistance. Nevertheless, the outcomes of randomized controlled trials evaluating the role of vitamin D supplementation on glucose homeostasis in PCOS patients remain inconclusive.[18] Vitamin D supplementation showed no effect on PCOS patients’ fasting glucose, serum HOMA-IR, fast insulin, or quantitative insulin sensitivity check index (QUICKI), according to a recent meta-analysis that involved only the findings of controlled randomized trials.[19]
Additionally, insulin receptor gene upregulation, modulation of insulin production from pancreatic β-cells, and promotion of β-cell proliferation and adiponectin production have all been demonstrated to enhance metabolic state by supplementation with vitamin D, vitamin K, and calcium.[20]
CONCLUSION
Vitamin D addition to induction of ovulation in women with PCOS resulted in enhancement of ovulation and pregnancy rates. Larger studies are still required to add to the evidence to reach to a solid conclusion regarding this issue.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-55.
- [Google Scholar]
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41-7.
- [Google Scholar]
- Treatment strategies for women with polycystic ovary syndrome. Gynecol Endocrinol. 2018;34:272-7.
- [Google Scholar]
- Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2011;95:1073-9.
- [Google Scholar]
- Vitamin D in the aetiology and management of polycystic ovary syndrome. Clin Endocrinol (Oxf). 2012;77:343-50.
- [Google Scholar]
- Vitamin D and cardiometabolic risk factors and diseases. Minerva Endocrinol. 2015;40:213-30.
- [Google Scholar]
- Vitamin D, PCOS and androgens in men: a systematic review. Endocr Connect. 2018;7:R95-113.
- [Google Scholar]
- Vitamin D (3rd). New York, USA: Elsevier Academic Press; 2011.
- Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch Gynecol Obstet. 2009;280:559-63.
- [Google Scholar]
- Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod. 2008;23(3):462-77.
- [Google Scholar]
- Effect of two different doses of vitamin D supplementation on metabolic profiles of insulin-resistant patients with polycystic ovary syndrome. Nutrients. 2017;9:1280.
- [Google Scholar]
- Effects of vitamin D supplementation on metabolic and endocrine parameters in PCOS: a randomized-controlled trial. Eur J Nutr. 2019;58:2019-28.
- [Google Scholar]
- The role of vitamin D oral supplementation in insulin resistance in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2018;10:1637.
- [Google Scholar]
- The effects of vitamin D-K-calcium co-supplementation on endocrine, inflammation, and oxidative stress biomarkers in vitamin d-deficient women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Horm Metab Res. 2016;48:446-51.
- [Google Scholar]
- Role of vitamin D supplementation therapy on ovulation and insulin resistance in women with PCOS: a randomized controlled trial. Evid Based Women’s Health J. 2019;9:329-6.
- [Google Scholar]
- Efficacy of high dose vitamin D3 supplementation in improving serum 25 (OH) D levels of overweight women with polycystic ovary syndrome: randomized placebo-controlled clinical trial. Act Sci Nutr Health. 2018;2:22-7.
- [Google Scholar]
- Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol. 2009;161:575-82. doi: 10.1530/EJE-09-0432
- [Google Scholar]
- Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin Nutr. 2015;34:586-92.
- [Google Scholar]







