Translate this page into:
Free Testosterone and Percentage Free Testosterone are Superior Marker than Total Testosterone in Evaluation of Male Infertility—A Case-control Study

*Corresponding author: Dr. Waseem Andrabi, PhD, Cluster Head, Embryology, Department of Embryology, Nova IVF Fertility, Delhi, India. wasiandrabi@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Andrabi W, Singh SK, Makker GC, Kumari K, Hashmi T, Makker R, Malik S. Free Testosterone and Percentage Free Testosterone are Superior Marker than Total Testosterone in Evaluation of Male Infertility—A Case-control Study. Fertil Sci Res. 2025;12:3. doi: 10.25259/FSR_17_2024
Abstract
Objectives
To evaluate the hormonal profiles, specifically focusing on gonadotropins (FSH, LH) and testosterone levels (Total, free and Percentage free Testosterone), in infertile males (oligozoospermia and azoospermia) compared to fertile controls, and which of these hormones provide a better clinical relevance during evaluation of male reproductive health.
Material and Methods
Study design-A retrospective study in which a total of 250 infertile male patients were studied and categorized into: 172 patients with oligozoospermia (68.8%), 78 patients with azoospermia (31.2%) and 52 fertile males included as a control group (proven fertile males). Blood samples were collected from all participants to measure serum levels of Follicle-stimulating hormone (FSH), Luteinizing hormone (LH), Estradiol, Prolactin Total testosterone, Free testosterone and Percentage Free testosterone along with controls and a comparison of hormonal levels between infertile patients (both oligozoospermic and azoospermic) and the control group was performed using appropriate statistical methods to check significance.
Results
Significant differences were observed in the levels of FSH, LH, free testosterone, and percentage free testosterone between the oligozoospermic and azoospermic patients when compared to the control fertile group. However, no significant differences were found in estradiol, prolactin, and total testosterone levels between the oligozoospermic and azoospermic patients compared to the control fertile males.
Conclusion
The study highlights the critical role of hormones in male fertility. The significant alteration in FSH, LH, free testosterone levels and percentage free testosterone levels in infertile men suggests that hormonal evaluation is crucial for understanding and diagnosing male infertility. Anomalies in these hormones may contribute to the pathophysiology of spermatogenic dysfunctions, emphasizing the importance of endocrine function in male reproductive health.
Keywords
Azoospermia
Free testosterone
FSH
LH
Oestradiol
Oligozoospermia
Percentage free testosterone
Prolactin
Total testosterone
INTRODUCTION
Infertility is defined by the American Society of Reproduction and Medicines (ASRM) as the inability of a couple to conceive after one year of unprotected sexual intercourse. There are numerous causes for infertility issues, the issues with either the male or female factor, and sometimes both can be the reason. The cases where no obvious cause is found are termed unexplained infertility. Male infertility contributes equally to that of female infertility in 15–20% of couples suffering from infertility issues worldwide.[1] Hormones play a very important role in reproduction by initiating and maintaining the reproductive function.
The proper endocrine interaction of the hypothalamus, pituitary and testis results in a healthy and complete germ cell development in males. Androgens, necessary for the development and growth of genitals, also play a part in the physiology of erection.[2] Spermatogenesis starts right after the differentiation of spermatogonial stem cells in an environment that is hormonally controlled in the testes. From the hypothalamus, the gonadotropin-releasing hormone (GnRH) is secreted in a pulsatile manner, which stimulates the two main glycoprotein hormones, follicle-stimulating hormone (FSH) and luteinising hormone (LH) in the anterior lobe of the pituitary gland.
The disruption in spermatogenesis is caused by many factors. One of the causes can be the absence or deficiency of gonadotropins (FSH and LH) from the pituitary. The secretions of these hormones are controlled by testosterone, inhibin and oestradiol. The testosterone, along with estradiol-17β, is associated with hormonal regulation [3,4] supervised by the hypothalamus–pituitary–testicular axis under the influence of FSH and LH. Apart from these hormones, inhibins, activins and various paracrine factors also participate.
The FSH regulates spermatogenesis when it activates signalling pathways while acting on Sertoli cells along with testosterone. In the Sertoli cells, FSH plays an important role before and after puberty in proliferation and activation, respectively, which is essential for germ cell development. The indirect involvement of FSH in spermatogenesis is important in induction and maintenance.[5] The stimulation for the proliferation of Sertoli cells and final cell number is carried out by FSH, which in turn determines the size of seminiferous tubules and the testes. In spermatogonia and pre-leptotene spermatocytes, the stimulus for the mitotic and meiotic DNA synthesis is performed by FSH. Testicular function can be disrupted in case the pituitary fails to secrete FSH and LH, which can lead to infertility. Gonadotropin secretions are controlled by testosterone, oestradiol and inhibin.[6] The indication of seminiferous tubule damage has been seen in azoospermic and severe oligozoospermic infertile males with increased FSH.[7]
Testosterone production by Leydig cells is stimulated by the action of LH. The expression of receptors [luteinising hormone/choriogonadotropin receptor (LHCG or LHR)] essential for the production of testosterone starts at the foetal stage, and any mutations causing inactivation of the LHR cause testosterone deficiency.[8] The essential role in spermatogenetic initiation and maintenance by LH–testosterone signalling has already been authenticated in various animal models.[9] Leydig cells at the foetal stage express receptors known as luteinising hormone/choriogonadotropin receptor (LHCG or LHR). Upon binding of LH, the Leydig cells are stimulated for testosterone production, which is its primary function. However, this complex stimulation process involves the aid of different types of somatic cells and signalling pathways that operate indirectly. Several transcription factors and steroidogenic enzyme genes are expressed by LH to stimulate and synthesise testosterone necessary to carry out reproductive function. In humans, mutations leading to the inactivation of LHR result in testosterone deficiency and problems in genitalia development, emphasising the importance of LH signalling in testosterone production.[8] An elective lifestyle also affects the LH-mediated production of testosterone in human foetal testes, as the sensitivity of steroidogenesis to environmental toxins is very high.[10]
On the other hand, low testosterone levels affect the number of erections and maintenance along with firmness. Improvements in the above parameters have been shown by administrating the testosterone levels.[11] The production of testosterone is inhibited by oestradiol administration, which can lead to erectile dysfunction. A reduction in spontaneous erections has been found in males taking oestradiol.[12] Males with low testosterone levels have been found to have high levels of oestradiol.[13]
MATERIAL AND METHODS
In this study, we prospectively screened 650 infertile men between 18 and 51 years of age referred to the tertiary infertility clinic between March 2015 and September 2019. A total of 250 infertile males were included in this study, of which 172 were severe oligozoospermic, and 78 were non-obstructive azoospermic. A group of 52 fertile men were included as controls. An informed consent was obtained from all participants recruited for the study. Patients who were undergoing hormonal therapy were not included, and the testicular evaluation results and detailed history were recorded.
In our case-control study, we selected an exposed proportion (P) of 0.05, which indicated that 5% of the control group was exposed to the risk factor. We determined that a minimum sample size of 25 participants per group (cases and control) is required.
Semen analysis
A semen analysis assessment was performed after the abstinence period (minimum 2 days and maximum 5 days). Following the WHO guidelines 2010, seminal volume, concentration, motility and progressive motility analyses were performed. Males with a sperm concentration of less than 5 million/mL were termed as “severe oligozoospermic.” They were termed “azoospermic” if no sperms were found after centrifugation of whole semen samples collected on two different occasions in 2 months. A thorough clinical physical evaluation was performed for every azoospermic male, as described in Figure 1.
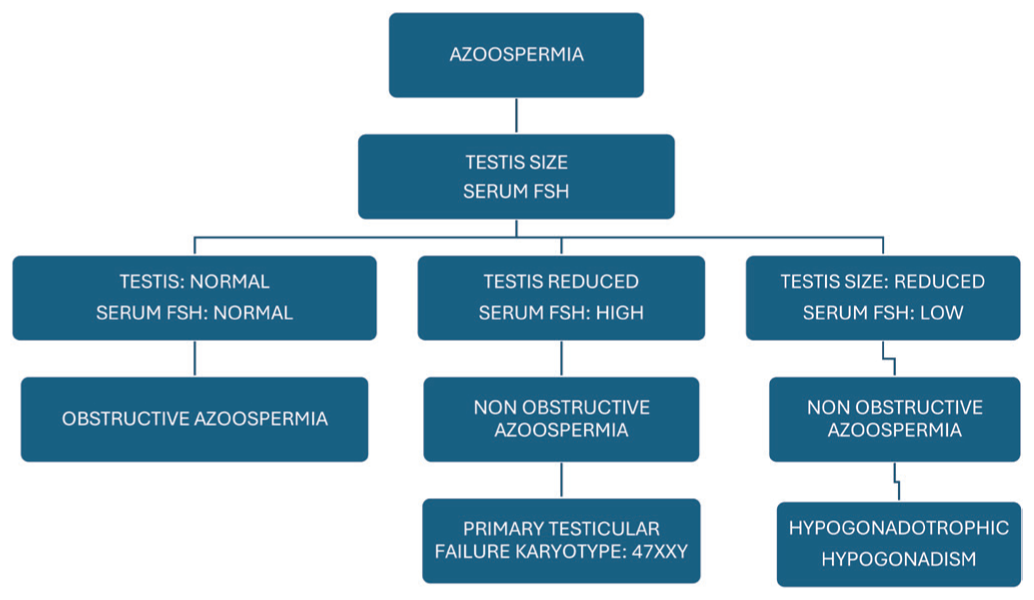
- Evaluation of azoospermia patients. FSH: Follicle-stimulating hormone
Hormonal evaluation
Blood samples were drawn from all participants in the morning after overnight fasting. The samples were allowed to clot for half an hour and then centrifuged for 15 minutes at 5000 rpm. To avoid bioactivity loss and contamination, serum from the supernatant was collected and stored at −20°C. Serum FSH, LH, total testosterone, free testosterone, percentage free testosterone, oestradiol and prolactin levels were estimated using electrochemiluminescent immunoassay (ECLIA). Reference ranges for hormonal analysis were as follows: FSH 1.50–12.40 mIU/mL, LH 1.7–8.6 mIU/mL, total testosterone 2.8–8.0 ng/mL, free testosterone 3.84–34.17 pg/mL, percentage free testosterone 0.18–0.68 pg/mL, oestradiol 25.8–60.7 pg/mL and prolactin 4.6–12.40 ng/mL.
Statistical analysis
The statistical data analysis was performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA) and R environment ver.3.2.2 software. The chi-square or Fisher exact test was used to find the significant differences and frequency distribution. Analysis of variance (ANOVA) was used to determine the significance of study parameters between three or more groups of patients. P-value < 0.05 was considered statistically significant.
RESULTS
A total of 302 participants, comprising 172 severe oligozoospermic, 78 azoospermic and 52 fertile control males, were included in the study. The clinical characteristics of the recruited participants are presented in Table 1.
| Group | No. of patients | % |
| Oligozoospermia | 172 | 57.0 |
| Azoospermia | 78 | 25.8 |
| Control | 52 | 17.2 |
| Total | 302 | 100.0 |
The mean ages of the subjects were as follows: 32.48 ± 6.51 years for severe oligozoospermic, 32.44 ± 5.78 years for azoospermic and 32.15 ± 5.80 years for controls [Table 2]. The cases and controls mainly fell under the age group of 20–40 years (91%).
| Diagnosis | Age in years |
| Oligozoospermia | 32.48 ± 6.51 |
| Azoospermia | 32.44 ± 5.78 |
| Normozoospermic | 32.15 ± 5.80 |
| Total | 32.41 ± 6.19 |
On further analysis, in oligozoospermic males, 2 were less than 20 years (1.2%), 66 were between 20 and 30 (38.4%), 90 were between 31 and 40 (52.3%), 12 were between 41 and 50 (7%) and 2 (1.2%) were above 50 years. In azoospermic males, 32 were between 20 and 30 years (41%), 40 were between 31 and 40 (51.3%), 6 were between 41 and 50 (7.7%) years, and no participants were below 20 and above 50 years of age. In control fertile males, 24 were between 20 and 30 years (46.2%), 23 were between 31 and 40 (44.2%), 5 were between 41 and 50 years (9.6%), and no participants were below 20 and above 50 years. After comparing the mean ± SD of age (in years) in oligozoospermic (32.48 ± 6.51), azoospermic (32.44 ± 5.78) and fertile male control (32.15 ± 5.80) groups, no significant difference was found between these groups (P = 0.945, Students t-test). All parameters are summarised in Table 3.
| Age in years | Oligozoospermia | Azoospermia | Control | Total |
|---|---|---|---|---|
| <20 | 2 (1.2%) | 0 (0%) | 0 (0%) | 2 (0.7%) |
| 20–30 | 66 (38.4%) | 32 (41%) | 24 (46.2%) | 122 (40.4%) |
| 31–40 | 90 (52.3%) | 40 (51.3%) | 23 (44.2%) | 153 (50.7%) |
| 41–50 | 12 (7%) | 6 (7.7%) | 5 (9.6%) | 23 (7.6%) |
| >50 | 2 (1.2%) | 0 (0%) | 0 (0%) | 2 (0.7%) |
| Total | 172 (100%) | 78 (100%) | 52 (100%) | 302 (100%) |
| Mean ± SD | 32.48 ± 6.51 | 32.44 ± 5.78 | 32.15 ± 5.80 | 32.41 ± 6.19 |
SD: Standard deviation.
The mean values for serum LH, FSH, total testosterone, free testosterone, percentage free testosterone, prolactin and oestradiol are summarised in Table 3. Total testosterone between the three categories was found to be non-significant. The mean serum concentration of LH was found to be significantly higher in infertile azoospermic (9.60 ± 7.39 ng/mL) and oligozoospermic males (7.87 ± 5.73 ng/mL) compared to the fertile controls (5.16 ± 1.92 ng/mL) (P < 0.001). The FSH levels were also found to be significantly higher in azoospermic (19.78 ± 16.79) and severe oligozoospermic (11.34 ± 12.27) males compared to the controls (3.28 ± 1.29) (P < 0.001). Total testosterone levels were found to be non-significant (P < 0.104). The difference in free testosterone (P < 0.006) and percentage free testosterone (P < 0.001) levels were statistically significant between the three groups. The mean serum prolactin level was higher among infertile azoospermic (24.70 ± 90.08 ng/mL) and oligozoospermic males (26.38 ± 96.29 ng/mL) than controls (24.70 ± 90.08 ng/mL) but was statistically non-significant (P > 0.05, ANOVA test). Similarly, oestradiol levels were found to be non-significant between the cases and controls. These parameters are described in Table 4.
| Variables | Oligozoospermia | Azoospermia | Control | P |
|---|---|---|---|---|
| LH | 7.87 ± 5.73 | 9.60 ± 7.39 | 5.16 ± 1.92 | <0.001 |
| FSH | 11.34 ± 12.27 | 19.78 ± 16.79 | 3.28 ± 1.29 | <0.001 |
| Testosterone total | 16.58 ± 66.64 | 4.37 ± 2.94 | 3.62 ± 1.66 | 0.104 |
| Testosterone free | 11.72 ± 13.59 | 9.12 ± 5.40 | 6.55 ± 3.41 | 0.006 |
| Percentage free | 0.23 ± 0.10 | 0.22 ± 0.07 | 0.40 ± 0.19 | <0.001 |
| Prolactin | 26.38 ± 96.29 | 24.70 ± 90.08 | 24.70 ± 90.08 | 0.977 |
| Estradiol | 36.24 ± 74.90 | 36.54 ± 17.36 | 32.71 ± 14.29 | 0.918 |
LH: Luteinising hormone, FSH: Follicle-stimulating hormone.
DISCUSSION
Male factor infertility is equally responsible for the social stigma of infertility. About 15% of the male infertility cases are idiopathic. The male factor infertility is a multifactoraial disorder, wherein environmental, genetic and endocrinological disruptions are the leading causes. The qualitative and quantitative analyses of semen samples are performed to measure normal spermatogenesis in males. The proper functioning of hormones is very important for normal spermatogenesis, and any abnormality can result in abnormal spermatogenesis and infertility. Hormonal profiling is important after proper semen analysis in cases of oligozoospermia or azoospermia to determine the underlying cause. Infertility in males due to endocrinological disruptions is caused by the improper functioning and regulation of the hypothalamus-pituitary–gonadal axis leading to an imbalance in reproductive hormones. LH acts on Leydig cells and increases testosterone levels. FSH is required for spermatogenetic initiation and is an important hormone for clinical and therapeutic interventions in infertile males, while intercellular testosterone stimulates Sertoli cells and increases spermatogenesis.
The majority of the participants in our study fell under the age group of 30–40 years, which was similar to that of another study where the majority of the patients were in the age group of 25–40 years.[14] Also, a majority of our study participants were oligozoospermic, which was similar to a previous study conducted in Indian patients, in which the frequency of oligozoospermia was higher.[15]
Significant elevated levels of serum LH and FSH hormones were found in infertile males with oligozoospermia and azoospermia when compared to normozoospermic fertile males, which supported the finding from a previous study that indicated the role of hormones in abnormal spermatogenesis.
Various studies have been carried out to determine the effects of hormones on male fertility by examining the FSH, LH and testosterone levels. In one such study carried out by Gangwar et al., serum FSH was found to be significantly higher in infertile oligozoospermic patients compared to the fertile controls; however, serum FSH and testosterone levels were non-significant between the two groups.[16] In another study, FSH was favoured as a diagnostic marker in infertile males.[17]
Our study evaluated and investigated the hormonal parameters of a total of 250 infertile males, comprising 78 azoospermic and 172 oligozoospermic cases, who attended the fertility clinic between 2015 and 2019. The levels of both FSH and LH were found to be significantly higher in infertile men (P < 0.001) when compared to fertile participants, thus suggesting their roles in fertility. However, no significant difference was found in the total testosterone levels between infertile and fertile males (P = 0.014). Further validation and studies are required to explore more on this issue further.
CONCLUSION
Hormonal analysis is an important test performed in all fertility clinics. In males whose semen parameters are very low or in whom semen is absent, semen analysis is an important and basic gold standard test for checking fertility. Those with an absence of sperm or a low sperm count are subjected to hormonal analysis, where tests to analyse the levels of LH, FSH, testosterone and estradiol are done routinely. Not all physicians give importance to additional tests such as free testosterone and percentage free testosterone, which are, in fact, equally important. Our study is a forward leap to determine the significance of free testosterone and percentage free testosterone levels as biomarkers in infertile patients, especially azoospermic and oligospermic males.
Author contributions
WA: Responsible for writing the manuscript, contributing to the overall structure, and ensuring clarity of the research findings; SS: Engaged in writing sections of the manuscript, focusing on specific details related to the study’s methodology and outcomes; GCM: Contributed to the clinical discussion, providing insights and expertise that informed the interpretation of results; also assisted in data analysis; KK: Played a key role in managing data, ensuring accurate collection, organization, and integrity of the research data; TH: Involved in data management, assisting in the collection and processing of data relevant to the study’s objectives; RM and SK: Led the discussion section of the paper, synthesizing the findings and drawing conclusions based on the analysed data.
Ethical approval
Ethical approval has been taken from the Institutional Review Board.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent and patient cofidentiality has been maintained while handling the data.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
Use of artificial intelligence (AI)–assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)–assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
REFERENCES
- Human Chorionic Gonadotropin Therapy in Hypogonadic Severe-oligozoospermic Men and Its Effect on Semen Parameters. Clin Exp Reprod Med. 2022;49:57-61.
- [Google Scholar]
- Androgen Physiology, Pharmacology, Use and Misuse. [Updated 2020 Oct 5]. In:. In: Feingold KR, Anawalt B, Blackman MR, eds. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279000/ [Last accessed on 2024 Jun 25]
- [Google Scholar]
- The Role of Estradiol in Male Reproductive Function. Asian J Androl. 2016;18:435-40.
- [Google Scholar]
- The Impact of 17β-estradiol on the Estrogen-deficient Female Brain: From Mechanisms to Therapy with Hot Flushes as Target Symptoms. Front Endocrinol (Lausanne). 2024;14:1310432.
- [Google Scholar]
- Role of Follicle-Stimulating Hormone in Spermatogenesis. Front Endocrinol (Lausanne). 2018;9:763.
- [Google Scholar]
- Physiology of GnRH and Gonadotrophin Secretion. [Updated 2024 Oct 15]. In:. In: Feingold KR, Anawalt B, Blackman MR, eds. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279070/ [Last accessed on 2024 Jun 25]
- [Google Scholar]
- Male Infertility. [Updated 2024 Feb 25]. In:. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562258/ [Last accessed on 2024 Jun 25]
- [Google Scholar]
- The Roles of Luteinizing Hormone, Follicle-Stimulating Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited. Int J Mol Sci. 2021;22:12735.
- [Google Scholar]
- The Role of Testosterone in Spermatogenesis: Lessons from Proteome Profiling of Human Spermatozoa in Testosterone Deficiency. Front Endocrinol (Lausanne). 2022;13:852661.
- [Google Scholar]
- Effect of Environmental and Pharmaceutical Exposures on Fetal Testis Development and Function: A Systematic Review of Human Experimental Data. Hum Reprod Update. 2019;25:397-421.
- [Google Scholar]
- Erectile Dysfunction. [Updated 2024 Jan 9]. In:. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562253/ [Last accessed on 2024 Jun 25]
- [Google Scholar]
- Effect of Estradiol on Penile Erection: A Cross-sectional Study. Transl Androl Urol. 2019;8:574-82.
- [Google Scholar]
- Impact of the Association Between Elevated Oestradiol and Low Testosterone Levels on Erectile Dysfunction Severity. Asian J Androl. 2013;15:492-6.
- [Google Scholar]
- Prevalence of Male Factor Infertility in Last Ten Years at a Rural Tertiary Care Centre of Central India: A Retrospective Analysis. Indian J Obstet Gynecol Res. 2015;2:132-6.
- [Google Scholar]
- Increased Gonadotropins and Prolactin are Linked to Infertility in Males. Bioinformation. 2020;16:176-82.
- [Google Scholar]
- The Impact of Male Factors and Their Correct and Early Diagnosis in the Infertile Couple’s Pathway: 2021 Perspectives. J Endocrinol Invest. 2022;45:1807-22.
- [Google Scholar]








