Translate this page into:
Independent effect of body mass index on clinical pregnancy rate in single blastocyst frozen embryo transfer cycle in Asian women
Address for correspondence: Dr. Tejashri M. Shrotri, M.B.B.S., M.S., Center for IVF & Human Reproduction, Sir Gangaram Hospital, New Delhi, India. E-mail: drtejashrisb@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
There are limited studies in literature regarding the effect of body mass index (BMI) on clinical pregnancy rate following frozen-thawed embryo transfers.
Objective:
We aim to study the independent effect of BMI on clinical pregnancy rate in single blastocyst frozen-embryo transfer cycle in Asian women.
Material and methods:
It is a prospective observational study involving 167 women who underwent single, good quality frozen-blastocyst transfer following a uniform protocol. Stimulation was done by hormone replacement therapy in all cycles. The study population was divided into various cohorts as per the BMI classification for Asian adult population (normal: 18.5–22.9, pre-obese: 23–24.9, obese I: 25–29.9, obese II: ≥30).
Results:
The positive β-HCG rate was 48.93% in normal BMI women, 55.31% in pre-obese, 50% in obese I, and 38.4% in obese II BMI groups. The clinical pregnancy rate was 36.17% in normal BMI women, 48.93% in pre-obese, 45% in obese I, and 30.07% in obese II BMI groups. The difference between various BMI subgroups as regards to positive β-HCG as well as clinical pregnancy is not statistically significant. Women with BMI ≥ 30 had numerically low clinical pregnancy rate, in comparison to those with lower BMI sub-groups. However, this difference was not statistically significant.
Conclusion:
This study concludes that BMI did not affect clinical pregnancy rate among women following a uniform protocol, single good quality frozen blastocyst transfer in Asian women. The increased difficulty during transfer for women with higher BMI suggests that body habitus may be responsible for difficult transfers, although this may not translate into a worse clinical pregnancy rate. A study with larger sample size may be needed to confirm these findings.
Keywords
Asian
body mass index
frozen-thawed embryo transfer
INTRODUCTION
Embryo cryopreservation has revolutionized the practice of assisted reproductive techniques (ART). It has helped the ART clinicians to minimize the two major complications of assisted reproductive cycles namely, Ovarian Hyperstimulation Syndrome (OHSS) and multiple pregnancy.[1] Vitrification has further modified the technique of cryopreservation by improving the survival of frozen-thawed embryos and thus enhancing the clinical pregnancy rates.[2,3] Vitrification has also made it possible to transfer an euploid single embryo in a synchronized endometrium and thus maximizing the chance of conception and live birth[2] while minimizing the overall cost of the treatment. Considering the multiple advantages of the cryopreserved embryo transfer cycles, it has now become imperative to concentrate on the finer modifications that could possibly improve the outcome of these cycles.
There is a growing interest among infertility clinicians to study the impact of increased body mass index (BMI) on pregnancy outcomes following ART. The majority of the available literature is regarding fresh embryo transfers[4,5,6] and there is a paucity of literature regarding effect of BMI on frozen-thawed blastocyst transfers.[7] Even the available studies provide conflicting results regarding the effect of BMI. Though some studies have advocated excluding women with high BMI above a particular threshold from in vitro fertilization (IVF) treatment,[8,9] a systematic review on this topic found there was a lack of sufficient data to support this practice.[10] As the prevalence of obesity increases, there is a growing body of evidence to suggest that elevated BMI adversely affect female reproductive health.[11] However, the effect of BMI on frozen-thawed embryo transfer (FET) cycles still remain unclear. With this background, we intend to study the independent effect of BMI on clinical pregnancy rate in single blastocyst frozen embryo transfer cycle.
AIMS AND OBJECTIVES
To assess the independent effect of BMI on clinical pregnancy rate in single blastocyst frozen embryo transfer cycle.
MATERIALS AND METHODS
It was a prospective observational study carried out between August 2018 and January 2019 at the Center for IVF and Human Reproduction at Sir Gangaram Hospital, New Delhi, India, a tertiary care referral center. After ovarian stimulation with antagonist protocol and oocyte retrieval, fertilization of oocytes was done either by IVF or intracytoplasmic sperm injection (ICSI) procedure as per the laboratory protocol. The embryos which were produced were cultured to blastocyst stage in sequential culture media (Vitrolife, Sweden).
Blastocyst grading was done on day 5 or 6 based on Gardner and Schoolcraft’s grading system.[12]
The vitrification procedure was used for embryo cryopreservation. Vitrification was carried out as per the suggested protocol by the manufacturer (Vitrolife, Sweden). All FET cycles with embryo frozen at blastocyst stage in which HRT was used as a method for endometrial preparation were included in the study.
Exclusion criteria
Patients with age > 40 years (at the time of freezing) and >45 years (at the time of transfer).
Untreated uterine factors.
Untreated hydrosalpinx.
All donor oocyte/embryo cycles.
Previous two failed FETs.
Endometrial preparation method other than HRT used.
A total of 167 cases of frozen embryo transfer were identified matching the inclusion criteria. The study population was divided into various cohorts as per the BMI classification for Asian adult population.[11] It is summarized in Table 1.

Endometrial preparation
A basal measurement of estradiol and progesterone levels were checked along with the endometrial thickness on day 2 or 3 of menses.
For preparing the endometrium, oral estradiol valerate 6–12 mg was given and continued until optimal endometrial thickness(≥7mm) or blood flows up to zone 3 or zone 4. Vaginal progesterone was then added and embryo transfer was performed after 5 days.
Embryos were thawed using the protocol as recommended by the manufacturer (RAPIDWARMTM BLAST, Vitrolife, Sweden).
Embryo transfer procedure
A single embryo transfer was performed. Any difficulty in performing the procedure was noted. The patients in which either the stylet, tenaculum, or sound was used to facilitate the procedure were considered to be difficult or traumatic transfers.
Luteal phase support
Luteal phase support in the form of vaginal only, injectable plus vaginal, or oral plus vaginal progesterone was decided, as per the choice of the treating consultant. Both estradiol and progesterone were continued until the seventh gestational week. Clinical pregnancies were assessed at seven gestational weeks, at which time the presence or absence of heartbeat by ultrasound was done.
All patients enrolled in the study were prospectively followed up. A serum BHCG test was conducted 11 days after FET and was considered positive if the value was >50 IU/L. The clinical pregnancy was defined by the presence of gestational sac(s) and fetal heart activity on trans-vaginal sonography 3 weeks later.
The positive β-hCG rate was calculated as the ratio of number of patients having positive β-hCG results and the total number of frozen single blastocyst transfers. For calculating the clinical pregnancy rate we divided the total clinical pregnancies with the total number of blastocyst transfers. The biochemical pregnancy rate was calculated by subtracting the early pregnancy losses (no clinical pregnancy) from the total number of cases having positive hCG results.
Statistical analysis
The data were analyzed using SPSS version 25.0. The Student’s t-test was used for continuous variables and Chi Square test was utilized for categorical variables. P-value < 0.05 was considered significant.
RESULTS
We studied 167 single blastocyst FET cycles. The demographic factors like age at freezing, age at embryo transfer, etiology of infertility, and reason for freezing were noted among various BMI cohorts. All the cohorts were well matched with respect to the demographic factors. The results are tabulated in Table 2.
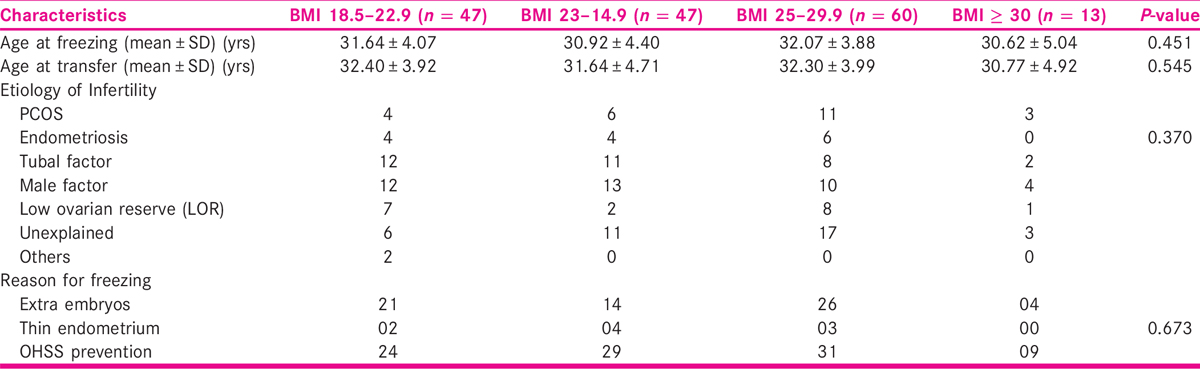
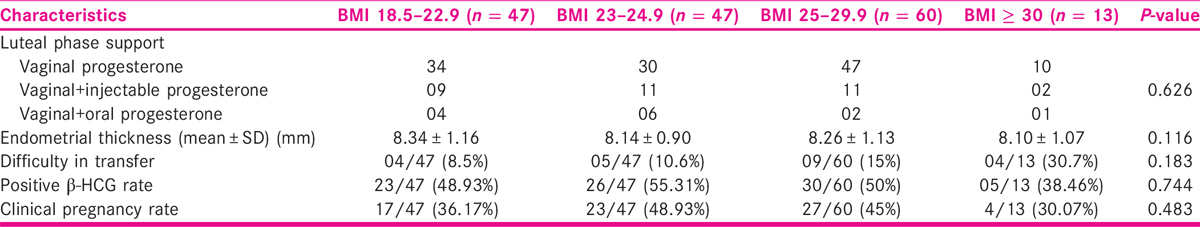
They were also well matched with respect to the luteal phase support received. The positive β-HCG rate and clinical pregnancy rate among various BMI cohorts are summarized in Table 3. It may be noted that women with BMI ≥ 30 had numerically low clinical pregnancy rate, in comparison to those with low BMI. However, this difference was not statistically significant, probably due to small sample size.
DISCUSSION
As ART techniques continue to evolve, with resultant improvement in IVF success rates,[13] some important physiological questions, such as whether BMI impacts fertility and pregnancy outcomes, still remain unanswered. Approximately half of reproductive-aged women in the west are overweight (BMI = 25.00–29.99 kg/m2) or obese (BMI ≥ 30.00 kg/m2); and this trend is almost becoming global. Thus, understanding this potential impact of BMI remains very important.[14,15] Compared to women with a normal BMI (18.50–24.99 kg/m2), women with an elevated BMI are more likely to experience disruption in the hypothalamic–pituitary–ovarian axis; irregular menstrual cycles, ovulatory dysfunction, as well as endometrial receptivity all of which lead to higher rates of infertility.[16] Following the advent of vitrification, nowadays many of the IVF centers across the world practice “freeze all” embryo strategy to prevent OHSS and multiple pregnancy. FET is increasingly being practised. Therefore, it is imperative to study whether BMI affects pregnancy outcome in FET cycles.
Some studies suggested that BMI did not affect IVF outcomes[17,18,19,20]; but there are other studies which seem to suggest the opposite.[5,6,15] Many of these studies are on IVF outcomes in fresh cycles. In one of the largest study based on 239,127 fresh autologous IVF cycles from 2008 to 2010 Society for Assisted Reproductive Technology registry,[5] the authors concluded that success rates in fresh autologous cycles, including those done for specifically PCOS or male-factor infertility, are highest in those with low and normal BMIs. Furthermore, there is a progressive and statistically significant worsening of outcomes in groups with higher BMIs. One of the most recent multicenter study[15] which analyzed more than 50,000 IVF cycles concluded that a BMI above the normal range was an independent negative prognostic factor for multiple outcomes, including cycle cancellation, oocyte and embryo counts, and ongoing clinical pregnancy. These negative outcomes were most profound in women with class-II/III obesity, ovulatory dysfunction, or PCOS.[15] These findings are also supported by a recent meta-analysis.[21]
Insogna et al.[7] studied effects of BMI on implantation rate after uniform protocol frozen-thawed blastocyst transfer in women with a homogenous uterine environment. They studied 461 infertile women who underwent standardized slow frozen-thawed blastocyst transfers with good quality day 5–6 embryos, following an identical hormonal uterine preparation, with comparison groups divided according to BMI category: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (>30.0 kg/m2). There were no statistically significant differences identified when comparing implantation rates among the four BMI cohorts. The implantation rate was 38.2% in normal weight patients, 41.7% in underweight patients, 45.1% in overweight patients, and 34.7% in obese patients. Adjusted odds ratios (OR) demonstrated no association between the main outcome, implantation rate, and BMI. Compared with the normal weight patients, the adjusted OR of implantation was 1.70 (95% confidence interval [CI], 0.40–7.72) for underweight patients, 1.61 (95% CI, 0.97–2.68) for overweight patients, and 0.92 (95% CI, 0.49–1.72) for obese patients. Secondary outcomes, including rates of miscarriage, clinical pregnancy, ongoing pregnancy, and live birth, were not significantly different between cohorts. We studied BMI subgroups among patients who were almost matched for age at freezing, age at transfer, etiology, reason for freezing as well as the choice of luteal phase support in the form of vaginal, injectable, or oral progesterone. Also we studied outcomes in HRT-stimulated cycles only to minimize confounders by creating a uniform uterine environment to assess the independent effect of BMI on clinical pregnancy rate. The BMI subgroups were divided based on WHO BMI classification for Asian adults, to make this study more relevant in local context.
Similar to the results of the study by Insogna et al.,[7] our study also did not find any statistically significant difference in β-HCG positivity and clinical pregnancy rate among various BMI cohorts. As per our results, it does appear that in obese patients with BMI above 30, transfers may be more technically challenging probably due to body habitus, as evidenced by the higher percentage of cases with difficulty in transfer in patients with BMI more than 30. However, this difference was not found statistically significant, probably due to small sample size.
The strength of our study is the assessment of BMI as an independent effect by matching the rest of the variables. And the fact that this study has been done in frozen embryo transfer cycles regarding which there are very few studies in literature. Weakness of the study is the small sample size.
CONCLUSIONS
Our study conclude that BMI did not affect clinical pregnancy rate among women following a uniform protocol, single good quality frozen blastocyst transfer in Asian women. The increased difficulty during transfer for women with higher BMI suggests that body habitus may be responsible for difficult transfers, although this may not translate into a worse clinical pregnancy rate. A study with larger sample size may be needed to confirm these findings.
Financial support and sponsorship
Nil.
Conflicts of interest.
There are no conflicts of interest.
REFERENCES
- Embryo freezing for preventing ovarian hyperstimulation syndrome. 2007;18:CD002806.
- Vitrification: a simple and successful method for cryostorage of human blastocysts. Methods Mol Biol. 2015;1257:305-19.
- [Google Scholar]
- The success rate in Swedish in-vitro fertilization unit: a cohort study. Acta Obstet Gynecol. 1995;74:446-50.
- [Google Scholar]
- A comprehensive analysis of body mass index effect on in vitro fertilization outcomes. Nutrients. 2016;8:109.
- [Google Scholar]
- Pregnancy outcomes decline with increasing body mass index: analysis of 239, 127 fresh autologous in vitro fertilization cycles from the 2008-2010 Society for Assisted Reproductive Technology registry. Fertil Steril. 2016;105:663-9.
- [Google Scholar]
- Low body mass index compromises live birth rate in fresh transfer in vitro fertilization cycles: a retrospective study in a Chinese population. Fertil Steril. 2017;107:422-9.
- [Google Scholar]
- Neutral effect of body mass index on implantation rate after frozen-thawed blastocyst transfer. Fertil Steril. 2017;108:770-6.
- [Google Scholar]
- Prioritising for fertility treatments—the effect of excluding women with a high body mass index. BJOG. 2006;113:1218-21.
- [Google Scholar]
- Prioritising for fertility treatments—should a high BMI exclude treatment? BJOG. 2006;113:1107-9.
- [Google Scholar]
- Complications and outcome of assisted repro- duction technologies in overweight and obese women. Hum Reprod. 2012;27:457-67.
- [Google Scholar]
- WHO/IASO/IOTF The Asia-Pacific perspective: redefining obesity and its treatment. Melbourne, Australia: Health Communications; 2000.
- Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155-8.
- [Google Scholar]
- In vitro fertilization (IVF): a review of 3 decades of clinical innovation and technological advancement. Ther Clin Risk Manag. 2006;2:355-64.
- [Google Scholar]
- The effect of female body mass index on in vitro fertilization cycle outcomes: a multi-center analysis. J Assist Reprod Genet. 2018;35:2013-23.
- [Google Scholar]
- The adverse effects of obesity on conception and implantation. Reproduction. 2010;140:347-64.
- [Google Scholar]
- Impact of body mass index on IVF and ICSI outcome: a retrospective study. Reprod Biomed Online. 2008;16:778-83.
- [Google Scholar]
- The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril. 2012;98:102-8.
- [Google Scholar]
- Effect of male and female body mass index on pregnancy and live birth success after in vitro fertilization. Fertil Steril. 2015;103:388-95.
- [Google Scholar]
- Effect of male and female body mass index on pregnancy and live birth success after in vitro fertilization. Fertil Steril. 2015;103:388-95.
- [Google Scholar]
- The correlation between raised body mass index and assisted reproductive treatment outcomes: a systematic review and meta-analysis of the evidence. Reprod Health. 2018;15:34.
- [Google Scholar]







