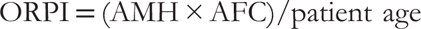Translate this page into:
Ovarian response prediction index (ORPI): A novel biomarker for ovarian response prediction in IVF cycle: An implication for individualized controlled ovarian stimulation programme
Address for correspondence: Dr Ritika Gupta, MBBS, DGO, DNB (Obstetrics and Gynecology), IFS, Diploma in clinical ART, A9/703, Tulip White, Sector 69, Gurugram, Haryana 122101, India. E-mail: ritikagupta128@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
The success of IVF depends to a large extent on the number and quality of mature oocytes obtained at the time of oocyte retrieval after controlled ovarian stimulation. This can be done by correctly predicting the ovarian response, which largely depends on ovarian reserve. The objective of present study was to assess accuracy of novel biomarker Ovarian Response Prediction Index (ORPI) based on three parameters patient age, AFC, AMH for prediction of ovarian response, so, we would be able to formulate individualized controlled ovarian stimulation approach to optimize cycle outcome.
Methodology:
This retrospective analysis was performed at our centre involving 100 patients undergoing IVF cycle between August 2019 and February 2020. We calculated ORPI by the following equation: ORPI = (AMH × AFC)/patient age, which was derived by Oliveira et al.
Results:
There was significant (p = 0.0001) positive correlation of ORPI with AFC, AMH, oocyte, MII oocyte and Embryo (gr1+2). ROC curve analysis showed that at ORPI cut off >0.18 (AUC 0.94) has sensitivity and specificity of 95.7% and 85.7%, respectively, for prediction of ≥4 oocyte recovery, and at cut off >2 (AUC 0.91) has sensitivity and specificity 75% and 85.2%, respectively, for prediction of ≥15 oocyte recovery. This study has demonstrated that for collection of ≥4 MII oocytes, ORPI at cut off >0.50 (AUC 0.86) has sensitivity and specificity of 74.1% and 78.9%, respectively. For probability of collection of >6 good quality embryos, we found ORPI cut off >0.75 with sensitivity and specificity of 72.7% and 64.2%, respectively.
Conclusion:
This study demonstrated that ORPI, which is simple 3 variable index, has shown excellent ability to predict low and excessive ovarian response and can be useful to tailor individualized controlled ovarian stimulation programme and will be beneficial for counselling and prognostication of infertile couple.
Keywords
Age
anti-Müllerian hormone
antral follicle count
individualised controlled ovarian stimulation
ovarian response prediction index
INTRODUCTION
The success of in vitro fertilization (IVF) depends to a large extent on the number and quality of mature oocytes obtained at the time of oocyte retrieval after controlled ovarian stimulation (COS).
The purpose of an optimized ovarian stimulation is to achieve good number of oocyte, simultaneously avoiding OHSS. In era of individualized COS, the correct optimization and individualization of the gonadotropin start dose is an extremely important clinical decision in IVF cycles. This can be done by correctly predicting the ovarian response. Ovarian response in COS largely depends on ovarian reserve. In other word, evaluation of the ovarian reserve is necessary to achieve an appropriate COS.[1]
There are myriad ways of checking ovarian reserve, among them most important is patient’s age. Although the number and quality of oocytes both decreases with age, the reproductive potential varies drastically among women of similar age.[2]
In fact, in addition to age, several clinical, endocrine, ultrasound markers and dynamic tests have been proposed for the prediction of the ovarian response to stimulation like AMH, basal follicle stimulating hormone (FSH), inhibin B, antral follicle count (AFC) and ovarian volume; or dynamic reserve tests like Gonadotropin Agonist Stimulation Test (GAST), Clomiphene Citrate Challenge Test (CCCT) and Exogenous FSH Ovarian Reserve Test (EFFORT).[3,4,5,6]
Among these markers, use of level of anti-Müllerian hormone (AMH) and the AFC is of particular interest.[7,8,9]
However, despite the predictive power that each marker for ovarian response may have individually, all these markers have errors associated with their estimation, none of them individually is reliable.
A systematic review of tests predicting ovarian reserve and IVF outcome observed that accuracy of the so-called ovarian reserve tests in predicting the occurrence of both a poor ovarian response and hyperstimulation appears to be modest. Therefore, prediction of the ovarian response using a single biomarker may not be sufficient for the formulation of a precise treatment plan.[7]
Ovarian Response Prediction Index (ORPI) was first determined by Oliveira et al. (2012), which is based on all three parameters AMH, AFC and age {ORPI= [AMH (ng/ml) × AFC (2–9 mm)/patient age] and showed an excellent ability to predict ovarian response.[10]
The objective of present study was to assess accuracy of ORPI for prediction of ovarian response, so that we would be able to formulate individualized COS approach to optimize cycle outcome.
The ORPI might be used to improve the cost benefit ratio of ovarian stimulation regimes by guiding the selection of medications and by modulating the doses and regimens according to patient’s need.
METHODOLOGY
This was a retrospective study of 100 patient undergoing IVF–ICSI cycle over period of August 2019–February 2020 at our institute.
Inclusion Criteria
Age ≤40 years
Body mass index (BMI) between 20 and 35 kg/m2
Both ovaries present
No history of ovarian surgery
No severe endometriosis
Exclusion Criteria
Presence of ovarian cysts as assessed by transvaginal ultrasound
Antral Follicle Count
Using TVS (Voluson S8) in early follicular phase, all ovarian follicles measuring 2–10 mm were counted and total for both ovaries was called as the basal AFC. The AFC correlates well with chronological age in normal fertile woman and appears to reflect what remains of the primordial follicular pool. A total AFC of <5 is predictive of poor ovarian response and higher cancellation rates with IVF.
AMH Measurement
A venous sample for AMH measurement was taken. AMH was assessed (Access AMH assay − chemiluminescent immunoassay) at any time of menstrual cycle. AMH is a glycoprotein dimeric hormone that belongs to the transforming growth factor beta (TGF-β) superfamily. It is expressed by the granulosa cells of primary, preantral and small antral follicles. It controls folliculogenesis by inhibiting the process of recruitment of primordial follicles and modifying the growth of preantral and antral follicles by diminishing the sensitivity of follicles to FSH.[11,12] The level of this hormone decreases with age, reaching undetectable levels in postmenopausal period since the pool of recruitable follicles goes on diminishing with age.[13,14]
Calculation of Ovarian Response Prediction Index
The ORPI was defined by the following equation
ORPI = (AMH × AFC)/patient age
This equation is based on previous evaluations that found that ovarian response to stimulation had positive correlations with AMH levels and number of antral follicles and was negatively correlated with patient’s age.[10]
Ovarian Stimulation Protocol
All patient enrolled for study underwent GnRH antagonist protocol. Gonadotropins were started with individualized doses on day 2/3 of menstrual cycle, called for follicular monitoring on day 6 of cycle, doses were adjusted depending on response. Antagonist (Inj. Cetrorelix 0.25 mg) was added when two or more follicle reached 14 mm diameter (flexible protocol), further follicular monitoring was done, hormonal testing was done as per need of patient. When 3 or more follicles reached 16–18 mm diameter, received 250 μg of recombinant hCG (Ovitrel; Merck Serono S.p.A.), oocyte retrieval done after 35 h of trigger.
End Points Measurement
End points measured were as follow: (1) total number of oocyte retrieved; (2) number of MII oocytes retrieved; (3) number of Day 3 cleavage stage good quality embryos (Day 3, Grade 1,2).
Statistical Analysis
The results are presented in frequencies, percentages and mean ± SD. The receiving operating curve (ROC) analysis was carried out. The area under the curve (AUC) with its 95% confidence interval (CI) was calculated. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) with its 95% CI was calculated. All the analyses were carried out on SPSS 16.0 version (Chicago, Inc., USA).
Observation and results
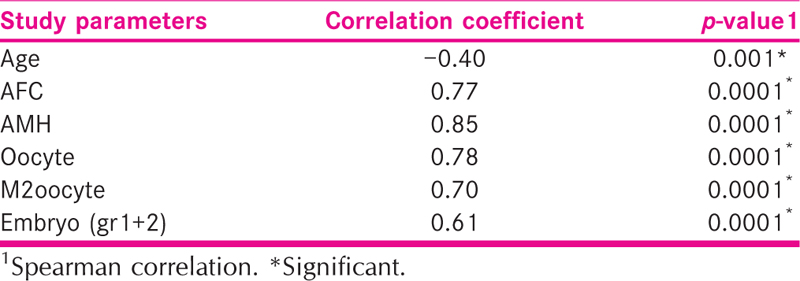
The mean age of patients was 30.21 ± 4.46 years ranging from 22 to 44 years. The mean AFC and AMH was 9.99 ± 4.51 and 3.65 ± 3.02, respectively. The mean number oocytes retrieved, MII oocyte and Embryos (Grades 1 +2) was 10.05 ± 5.16, 6.66 ± 4.17 and 5.28 ± 3.85, respectively. The mean ORPI was 1.34 ± 1.41 [Table 1].

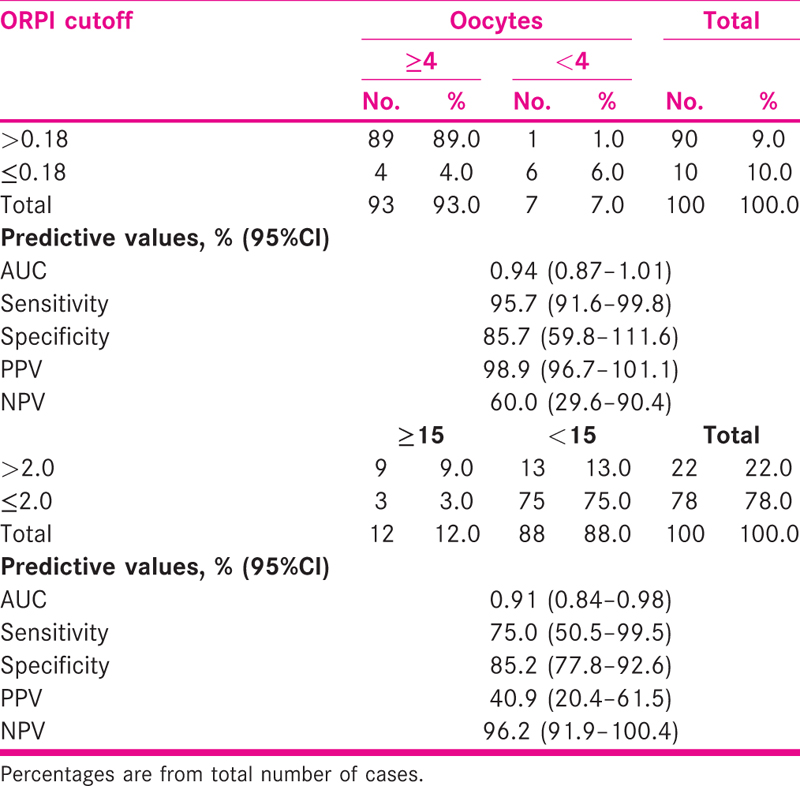
Oocyte ≥4 was among majority of patients (93%) and oocyte ≥15 was in 12% patients. MII oocyte ≥4 was in 81% patients. Embryo (gr1 + 2) 3–6 was among 48% patients [Table 2].

There was significant (p = 0.0001) positive correlation of ORPI with AFC, AMH, oocyte, MII oocyte and Embryo (gr1 + 2).
ORPI cut off >0.18 correctly predicted (efficacy) oocytes ≥4 among 89% with sensitivity and specificity of 95.7% (95%CI = 91.6–99.8) and 85.7% (59.8–111.6), respectively. The AUC was 0.94 (95%CI = 0.87–1.01). ORPI cut off >2 correctly predicted (efficacy) oocytes ≥15 among 9% with sensitivity and specificity of 75.0% (95%CI = 50.5–99.5) and 85.2% (95%CI = 77.8–92.6), respectively. The AUC was 0.91 (95%CI = 0.84–0.98) [Table 3 and Figs. 1 and 2].
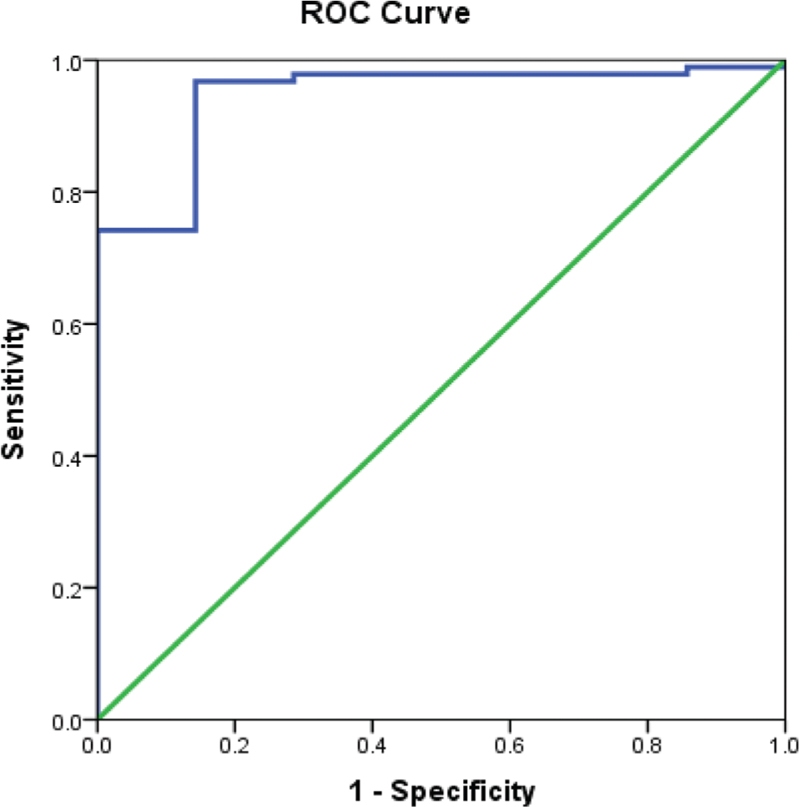
- ROC curve showing sensitivity and specificity of ORPI in predicting oocytes ≥4

- ROC curve showing sensitivity and specificity of ORPI in predicting oocytes ≥15
ORPI cut off >0.50 correctly predicted (efficacy) MII oocytes ≥4 in 60% with sensitivity and specificity of 74.1% (95%CI = 64.5–83.6) and 78.9% (60.6–97.3)m respectively. The AUC was 0.86 (95%CI = 0.78–0.94) [Table 4 and Fig. 3].
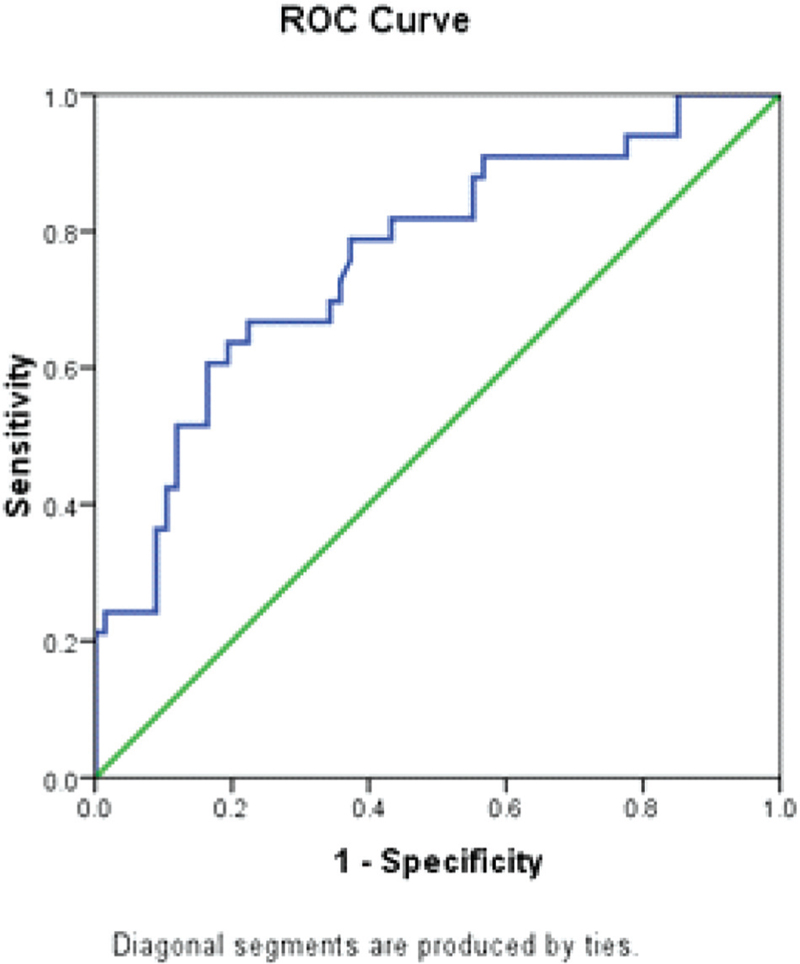
- ROC curve showing sensitivity and specificity of ORPI in predicting MII oocytes
ORPI cut off >0.75 correctly predicted (efficacy) embryo >6 in 24% with sensitivity and specificity of 72.7% (95%CI = 57.5–87.9) and 64.2% (52.7–75.7), respectively. The AUC was 0.76 (95%CI = 0.66–0.86) [Tables 5 and 6 and Fig. 4].
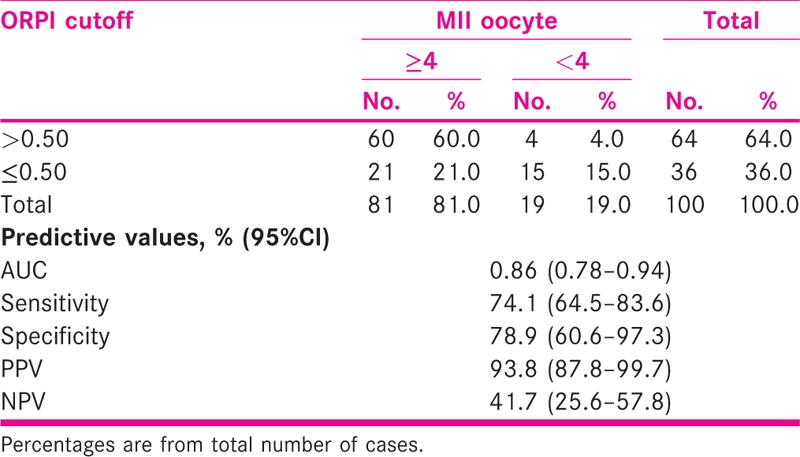
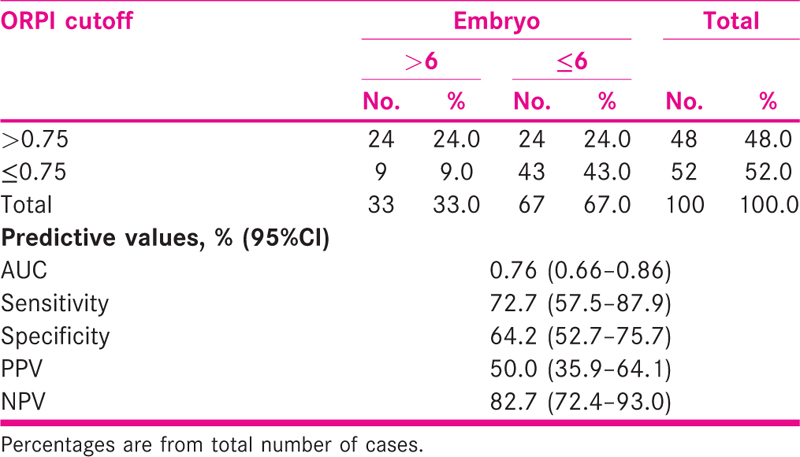
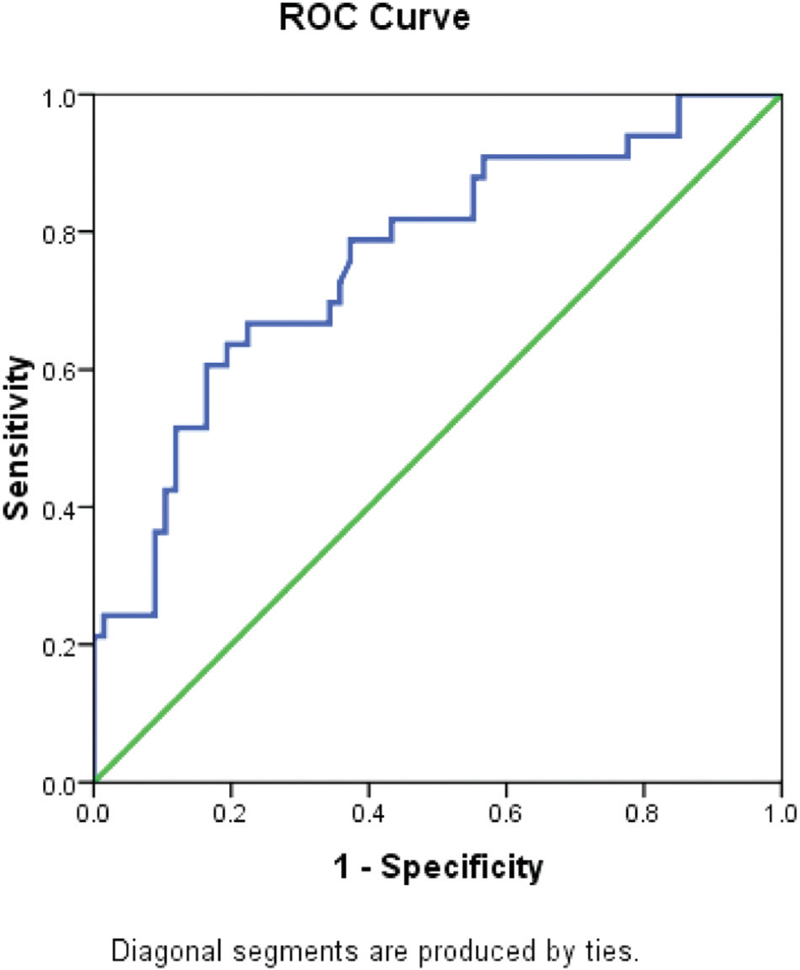
- ROC curve showing sensitivity and specificity of ORPI in predicting embryo
DISCUSSION
In spite of many advances in the field of reproductive medicine, the risk of poor ovarian response and excessive ovarian response following COS has remained as a strong obstacle in many programmes. A reliable indicator for providing more precise estimates of the patients’ ovarian response might facilitate the optimization and individualization of COS protocol before the onset of a treatment cycle. The European Society of Human Reproduction and Embryology Consensus Conference has established a standardized definition of poor ovarian response as the retrieval of <4 oocytes following a standard IVF protocol.[15] Similar to poor response, prediction of excessive response is also of great importance for the counselling and management of infertile women in IVF clinical practice. The “high response” is generally termed as the retrieval of >15oocytes following a standard COS protocol.[9] There is no definite rule and extremes of response may occur unexpectedly. As discussed in many review studies, there are different ways to predict ovarian response, most commonly used in practices are patient age, AFC, AMH, FSH value.
As a prognosticator of individual ovarian potential, chronological age is of limited value because women of the same age can be at different stages in the process of follicular depletion. This feature is also related to the wide range of age at the onset of menopause, which marks total follicular depletion.[16] Basal FSH has been shown to be a better marker of individual ovarian reserve than age,[17] and is to date commonly used in many infertility centres. But, it only has a moderate predictive performance for poor response. Predictions for absolute poor response are only achieved at extreme cut-off levels for basal FSH.[18]
AFC has been documented as a useful measure of ovarian reserve through various studies.[19,20,21] However, it is subjective and requires a high-resolution machine and reporting by the same observer to be an accurate marker of ovarian reserve. The dynamic reserve tests are time consuming, labour intensive and more expensive with no agreement on their endpoints. They do not add to the information obtained by static tests. Hence, there remains an unfulfilled need to establish an adequate test for predicting individual reproductive potential.
Serum concentrations of AMH, produced by granulosa cells of early follicles, are gonadotropin-independent and therefore remain relatively consistent within and between menstrual cycles.[22] Main drawbacks associated with AMH measurement are use of different commercially available assays and their different cut off values. Therefore, cut off points developed and reported for one commercial AMH assay are not generalizable to the other commercial assay(s). When applying AMH cut off points in clinical practice, one must be very careful to determine that the assay used to measure AMH is the same as that used in the reference study population. In addition, inappropriate storage and sample handling can cause a dramatic rise in the AMH level.[22,23,24] Level of AMH is influenced by many other factors like PCOS, oral contraception use, vitamin D levels.[25,26,27] Many studies have reported it to be useful marker of ovarian response, but no defined cut-off levels exist that help in deciding whether to enrol the patient for IVF or not. Also, it remains to be seen if the cut-off levels of AMH in Indian population are any different from those of the Caucasians.[28]Hence, reviewing literature, no individual test of ovarian reserve can accurately predict ovarian response in IVF cycle. There remains an unfulfilled need to establish an adequate test for predicting individual ovarian stimulation response. Idea of present study was to combine these three most reliable commonly used ovarian reserve maker (age, AFC, AMH) as ORPI and assessing its role in prediction of ovarian response to minimize drawback associated with individual test.
In present study, we have studied the role of novel ovarian response biomarker ORPI for prediction of recovery of number of oocyte, MII oocyte, good quality embryos in ovarian stimulation programme. We have found significant (p = 0.0001) positive correlation of ORPI with AFC, AMH, retrieved number of oocytes, MII oocytes and embryo (gr1 + 2). ROC curve analysis showed that ORPI >0.18 (AUC 0.94), this test has sensitivity and specificity of 95.7% and 85.7%, respectively, for prediction of ≥4 oocyte recovery; similarly, it found cut off >2 (AUC 0.91) for prediction of ≥15 oocyte recovery with sensitivity and specificity 75% and 85.2%, respectively.
Present study has demonstrated ORPI cut off >0.50 with sensitivity and specificity of 74.1% and 78.9%, respectively, for collection of ≥ 4 MII oocytes. The AUC was 0.86. Cut-off value for >15 M II oocyte could not be calculated due to limited data. For probability of collection of >6 good quality embryos, we found ORPI cut off >0.75 with sensitivity and specificity of 72.7% and 64.2%, respectively. The AUC was 0.76.
Results of present study were comparable to Oliveira et al.,[10] Kalpana et al.[29] Oliveira et al. has first given the concept this biomarker ORPI, demonstrated significant (P < 0.0001) positive correlations between the ORPI and the total number of oocytes and of MII oocytes collected. They demonstrated a cut-off of 0.2 with efficacy 88% for the probability of collecting greater than or equal to 4 and at a cut-off of 0.3 with efficacy of 81% for the probability of collecting greater than or equal to 4 MII oocytes. And for probability of collecting of >15 oocyte with cut-off 0.9 and efficacy of 82%. Regarding the probability of pregnancy occurrence according to the ORPI value, the ROC curve showed an AUC of 0.74 and an efficacy of 62% at a cut-off of 0.3.
CONCLUSION
This study demonstrated that ORPI, a novel ovarian response biomarker, which is simple 3 variable index has shown excellent ability to predict low and excessive ovarian response and can be useful to tailor individualized COS programme and will be beneficial for counselling and prognostication of infertile couple.
Limitation of study
It was a retrospective study with limited sample size and outcome measured was ovarian response. Clinical pregnancy rate and live birth rate was not taken in to consideration. Further lager studies are needed for confirmation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Age related decline in fertility: a link to degenerative oocytes. Fertil Steril. 1997;68:265-71. [PubMed] [Google Scholar]
- [Google Scholar]
- Hormonal, functional and genetic biomarkers in controlled ovarian stimulation: tools for matching patients and protocols. Reprod Biol Endocrinol. 2012;10:9. doi: 10.1186/1477-7827-10-9. Review. PubMed PMID: 22309877; PubMed Central PMCID: PMC3299595.
- [Google Scholar]
- Prognostic assessment of female fecundity. Lancet. 1989;2:645-7. [PubMed] [Google Scholar]
- [Google Scholar]
- Prognostic assessment of ovarian reserve. Fertil Steril. 1995;63:1-11. [PubMed] [Google Scholar]
- [Google Scholar]
- Prediction of individual response to controlled ovarian hyperstimulation by means of a clomiphene challenge test. Fertil Steril. 1990;53:295-301. [PubMed] [Google Scholar]
- [Google Scholar]
- A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685-718. Epub 2006 Aug 4. Review. PubMed PMID: 16891297.
- [Google Scholar]
- A prospective, comparative analysis of anti-Müllerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2010;93:855-64. [PubMed] [Google Scholar]
- [Google Scholar]
- Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16(2):113-30. doi:10.1093/humupd/dmp036. Epub 2009 Sep 30. Review. PubMed PMID: 19793843.
- [Google Scholar]
- A new ovarian response prediction index (ORPI): implications for individualised controlled ovarian stimulation. Reprod Biol Endocrinol. 2012;10:94. Published 2012 Nov 21. doi:10.1186/1477-7827-10-94
- [Google Scholar]
- Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143(3):1076-84. PubMed PMID: 11861535.
- [Google Scholar]
- The role of anti-Müllerian hormone in gonadal development. Mol Cell Endocrinol. 1998;145(1-2):3-7.
- [Google Scholar]
- Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81(2):571-6. PubMed PMID: 8636269.
- [Google Scholar]
- Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77-83. PubMed PMID: 14742691.
- [Google Scholar]
- ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26:1616-24.
- [Google Scholar]
- AMH and AFC as predictors of excessive response in controlled ovarian hyper stimulation: a meta analysis. Hum Reprod Update. 2011;17:46-54.
- [Google Scholar]
- Age and basal FSH as a predictor of ART outcome. Iranian J Reproduct Med. 2009;7(1):19-22.
- [Google Scholar]
- Age-specific serum antimullerian hormone levels in women with and without polycystic ovary syndrome. Fertil Steril. 2014;102(1):230-236.e2.
- [Google Scholar]
- Ovarian volume and antral follicle count for the prediction of low and hyper responders with in vitro fertilization. Reprod Biol Endocrinol. 2007;5:9. PubMed PMID: 17362511; PubMed Central PMCID: PM C1847823.
- [Google Scholar]
- Ultrasonography as a tool for the prediction of outcome in IVF patients: a comparative meta-analysis of ovarian volume and antral follicle count. Fertil Steril. 2007;87(4):764-75. Epub 2007 Jan 18. PubMed PMID: 17239869.
- [Google Scholar]
- The cohort of antral follicles measuring 2-6 mm reflects the quantitative status of ovarian reserve as assessed by serum levels of anti-Müllerian hormone and response to controlled ovarian stimulation. Fertil Steril. 2010;94(5):1775-81. doi: 10.1016/j.fertnstert.2009.10.022. PubMedPMID: 19931077.
- [Google Scholar]
- Biomarkers of ovarian response: current and future applications. Fertil Steril. 2013;99(4):963-9. doi: 10.1016/j.fertnstert.2012.11.051. Epub 2013 Jan 8. Review. PubMed PMID: 23312225.
- [Google Scholar]
- Use of anti-mullerian hormone for testing ovarian reserve: a survey of 796 infertility clinics worldwide. J Assist Reprod Genet. 2015;32(10):1441-8. doi:10.1007/s10815-015-0562-7. Epub 2015 Sep 7. PubMed PMID: 26347341; PubMed CentralPMCID: PMC4615913.
- [Google Scholar]
- Antimüllerian hormone as predictor of implantation and clinical pregnancy after assisted conception: a systematic review and meta-analysis. Fertil Steril. 2015;103(1):119-130.e3. doi:10.1016/j.fertnstert.2014.09.041. Epub 2014 Oct 24. Review. PubMed PMID: 25450298.
- [Google Scholar]
- Vitamin D and anti-Müllerian hormone levels in infertility treatment:the change-point problem. Nutrients. 2019;11(5):1053. Published 2019 May 10. doi:10.3390/nu11051053
- [Google Scholar]
- Effect of long-term using of hormonal contraception on anti-Müllerian hormone secretion. Gynecol Endocrinol. 2016;32(5):383. doi:10.3109/09513590.2015.1121981. Epub 2015 Dec 11. PubMedPMID: 26651155.
- [Google Scholar]
- The effect of vitamin D status on ovarian reserve markers in infertile women: a prospective cross-sectional study. Int J Reprod Biomed (Yazd). 2020;18(2):85-92. Published 2020 Feb 27. doi:10.18502/ijrm.v18i2.6501
- [Google Scholar]
- Anti-mullerian hormone cut-off values for predicting poor ovarian response to exogenous ovarian stimulation in in-vitro fertilization. J Hum Reprod Sci. 2012;5(2):206-12. doi:10.4103/0974-1208.101023. PubMed PMID: 23162361; PubMed Central PMCID. PMC3493837
- [Google Scholar]
- Modified ovarian response prediction index: a novel index for ovarian response prediction in GnRH agonist cycles. Int J Reprod Contracept Obstet Gynecol. 2019;8:2575-81.
- [Google Scholar]