Translate this page into:
Pregnancy after DHEA-S for low ovarian reserve due to laparoscopic ovarian drilling
Address for correspondence: Dr. Paapa Dasari, Department of OBGY, WCH, JIPMER, Puducherry -- 605006, India. E-mail: dasaripapa@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A 32-year-old married for three years, who had taken treatment for primary infertility, approached for assisted reproductive techniques (ART) after the failure of 10 cycles of ovulation induction with clomiphene citrate (CC) and laparoscopic ovarian drilling (LOD) two years ago. She was evaluated and found to have anti-Mullerian hormone (AMH) of 0.63 ng/ml and antral follicle count (AFC) of four and was treated with dehydroepiandrosterone sulphate (DHEA-S) for three months. Her AMH increased to 1.2 ng/ml and AFC to seven and she underwent three cycles of ovulation induction and intrauterine insemination (IUI) which was unsuccessful. She conceived naturally after the fourth cycle of ovulation induction with gonadotropins without cycle monitoring. Her Pregnancy was supported with progesterone until 36 weeks, and she underwent elective lower segment Caesarean section (LSCS) for mid-pelvic contraction at 38 weeks of pregnancy. The Caesarean section inspection of the ovaries showed bilateral charring and a few tubo-tubal adhesions. Her postoperative period was normal and mother and baby were discharged on the fifth postnatal day.
Keywords
Charring
DHEA-S
laparoscopic ovarian drilling
low ovarian reserve
INTRODUCTION
Management of fertility in a woman with low ovarian reserve is challenging. The option is intracytoplasmic sperm injection (ICSI) using pooled aspirated eggs of the same women employing various protocols like natural cycle, modified natural cycle, flare protocol or low dose human chorionic gonadotropin (HCG). Most often women with low ovarian reserve need oocytes donor. Treatment with DHEA-S is reported to increase ovarian pool and thus improve fertility. Literature in this regard is sparse. Therefore, this case study is important for reporting pregnancy and its outcome in a woman with low ovarian reserve who was treated with DHEA-S.
CASE
A 32-year-old hospital employee of our institute, who was married for three years, underwent fertility assessment after one year of marriage. Male factor was normal. Hysterosalphingogram (HSG) showed left cornual block. She received 10 cycles of ovulation induction with CC (clomiphene citrate), following which she also underwent hystero laparoscopy and ovarian drilling in 2015; bilateral tubal spillage was documented at that time.
Her past history included blood transfusion for anemia in 2007. She had a family history of diabetes and hypertension in father, and both her parents had expired. There were no marital issues. Husband was a software engineer who gave up alcoholism due to childlessness.
She sought ART in in July 2016.. Her menstrual cycles were normal occurring once 30 days lasting for five days. Her height was 150 cm, weight 44 kg, and BMI 19.5 kg/m2, Her secondary sexual characters were normal. Pelvic examination revealed normal cervix and uterus; there was no clinical evidence of PID (pelvic inflammatory disease)and no mass lesion in fornices. Her day-three FSH (follicle stimulating hormone) was 8.3, LH 3.3, serum prolactin 6.3 ng/ml, testosterone 2.1 ng/dl, and thyroid stimulating hormone (TSH) −1.47 mIU/L; her glucose tolerance test (GTT) was normal and AMH was 0.63 ng/ml. AFC was four and uterus was of normal size. She was diagnosed as poor ovarian reserve and advised to take oral DHEA 75 mg once daily for 3 months and review.
In January 2017, her AFC was seven and AMH was 1.1 ng/dl. As IVF services were not established at our center she was advised three cycles of IUI in the waiting period. First cycle of IUI was done in Feburary 2017 by employing CC+ antioxidants (Ovaa Shield) after HCG trigger at a follicular size of 22 mm. Ovulation was documented by TVS and luteal support was given with tablet Duphastan 10 mg once daily. In the second cycle with the same ovulogen she did not ovulate after HCG trigger on day 13 at a follicular size of 19 mm; the second trigger was given by injection Luprolide 1 mg. She developed LUF (luteinization of unruptured follicle) with follicle size of 28 × 17 mm in left ovary and 30 × 28 mm of another follicle in right ovary. There was a persistent cyst on day three of the next cycle in May 2017. She was advised to take tablet DHEA-S for a month. Third cycle IUI was done after ovulation induction with CC (100 mg) + Low dose hMG 75 IU on day five, seven, and nine. She developed two dominant follicles on each ovary and did not ovulate with HCG trigger given on day twelve at a follicular size of 20 mm and a double IUI was necessary after triggering with inj. Luprolide 1 mg im. Ovulation was documented after GnRH agonist trigger. In September 2017, CC + rFSH was used to induce ovulation and she did not come for cycle monitoring.
She came for check up on 12 December 2017, on the 47th day of amenorrhea with a positive pregnancy test report. TVS showed a single intrauterine gestational sac with yolk sac and a fetal pole with cardiac activity. She was administered luteal support with Inj HCG 10,000 IU weekly alongwith a transvaginal ultrasound (TVS). On 8 January 2018, live fetus with a Crown rump length (CRL) corresponding to 9 + 3 weeks was seen. Her Nuchal Translucency (NT) scan was within normal limits. The pregnancy was supported with inj HCG till 16 weeks. Her anomaly scan was normal.
She had regular antenatal care and was given progesterone support till 36 weeks and prophylactic antenatal corticosteroids at 28 weeks. Her growth scan was normal. USG at 37 and 3 days showed estimated fetal weight (EFW) of 2.7 kg with posterior placenta which had dense calcifications. AFI was 13 cm and umbilical artery doppler Pulsatality Index (PI) was 0.77 and non-stress test (NST) was reactive. Her weight gain during pregnancy was 10.3 kg. She underwent elective LSCS for mid-pelvic disproportion at 38 week of pregnancy under spinal anesthesia. A live male baby weighing 2.7 kg with Apgar score of 8/10 at 5 min was born. The uterus and tubes were normal. Both ovaries were small and normal in texture and had black (charred) areas with minimal tubo-ovarian adhesions as shown in Figure 1A and B. The postoperative course was normal, mother and baby were discharged on 5th postnatal day.
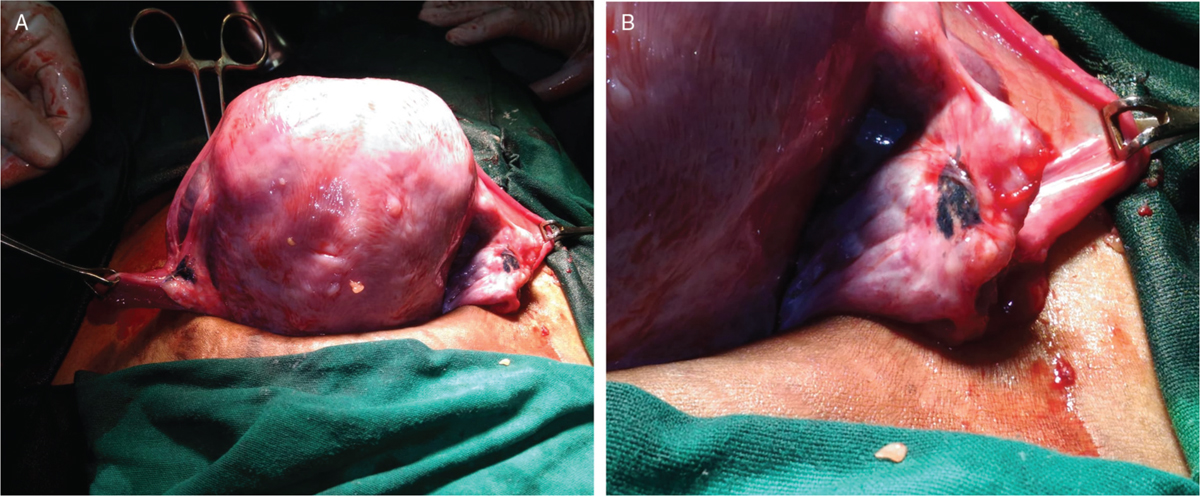
- (A) Posterior surface of uterus at Caesarean section showing bilateral adnexa with tubotubal adhesions of left fallopian tube and bilateral charring of ovaries. (B) Right adnexa showing the extensive charring as a black area.
DISCUSSION
Though Bologna criteria are used to diagnose poor ovarian reserve in ART, serum AMH has been designated as a quantitative marker for ovarian reserve and a level of <1 ng/ml is accepted as a low ovarian reserve in most studies. Ovarian reserve indicates the quantity and quality of the resting primordial follicles of the ovarian pool, which are necessary for recruitment to grow as graffian follicles. The causes of poor ovarian reserve include age-related decline, genetic factors such as mutation of Fragile X Mental retardation (FMR) gene, Fragile X-syndrome, autoimmune, impairment of adrenal gland function, iatrogenic such as oophorectomy, chemotherapy, ovarian drilling and idiopathic.[1] Poor ovarian reserve in this woman was most probably due to excessive inappropriate drilling due to ignorance of the procedure in an unindicated situation as evidenced by the gross features of ovaries at LSCS. Though LOD is reported to increase the ovulatory rates by increasing the sensitivity to ovulation inducing agents, it should be done only in selected CC resistant PCOS women and not in all women.[2] The rare complications of LOD are ovarian atrophy, DOR, and adhesions.[3] In this woman, the ovaries did not show any features of PCOS and they appeared charred due to cautery. The original study by Emad M Seyam and colleagues regarding assessment of ovarian reserve after LOD revealed a significant decrease from basal level in serum AMH levels after one week, three months, and six months period with correct technique of making four to five punctures in four to five second using 30 watt monopolar current in PCOS (polycystic ovarian syndrome) women. There was a significant ovarian response and also normalization of FSH/LH ratio. They concluded that LOD did not increase the risk of diminished ovarian reserve and normalized the Ovarian function in PCOS.[4]
Dehydroepiandrosterone is a natural hormone produced mainly by the zona reticularis adrenal cortex and theca cells of the ovary and is present in high concentration in adulthood and is therefore also known as “youth hormone.” Its therapeutic usefulness in women with diminished ovarian reserve was first reported by Casson and colleagues.[5] It is found to decrease the ROS, improve endothelial dysfunction, and increase endothelial cell proliferation. It increases the interleukin IL 1, IL6 levels. It mainly affects the IGF1 (Insulin like Growth Factor 1) levels and brings about changes in follicular growth and maturation. Androgen receptors are present in ovarian stroma, primordial follicles, primary follicles as well as advanced stages of follicles. Increased intrafollicular androgens are found to augment granulosa cell AMH and inhibin levels. The androgens are also responsible for an increasing the follicular recruitment in the ovary.[6]
A recent study conducted in France in a cohort of women with AMH <1.6 ng/ml undergoing IVF (327 cycles) reported significantly decreased cycle cancellation rates and increased clinical pregnancy rates.[7] An analysis of the studies done between 1995 and 2010 revealed DHEA to improve ovarian function, pregnancy rate, and decrease embryo aneuploidy and miscarriage rate.[8] A recent systematic review from 2000 to 2014 reported that DHEA improves pregnancy rates including the chances of spontaneous conception.[9]Recent studies have shown that its supplementation improves ovarian reserve markers, oocyte quality, and embryo quality. It is especially useful in women with poor ovarian reserve and women of more than 40 years of age[5,6]. Its efficacy and safety has been demonstrated by nonrandomized and a few randomized controlled studies and the evidence is accumulating regarding the role of DHEA in prior IVF failures.
CONCLUSION
This case illustrates the inappropriate management of a primary infertility and misuse of LOD in non PCOS woman resulting in low ovarian reserve. Low ovarian reserve due to LOD in a woman of under 35 years old may respond to DHEA-S therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Diminished ovarian reserve, causes, assessment and management. Int J Infertility Fetal Med. 2013;4:45-55.
- [Google Scholar]
- Laparoscopic ovarian drilling: An alternative but not the ultimate in the management of polycystic ovarian syndrome. J Nat Sci Biol Med. 2015;6(1):40-8.
- [Google Scholar]
- Evaluation of ultrasonographic and Antimullerian hormone (AMH) changes as predictors for ovarian reserve after laparoscopic ovarian drilling for women with polycystic ovarian syndrome. Middle East Soc Fertil J. 2014;19:314-23.
- [Google Scholar]
- Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000;15:2129-32.
- [Google Scholar]
- Increased intrafollicular androgen levels affect human granulose secretion of anti-Müllerian hormone and inhibin-B. Fertil Steril. 2008;89:1760-5.
- [Google Scholar]
- Preliminary Results of DHEA in poor responders in IVF. Open J Obstet Gynecol. 2016;6:396-403.
- [Google Scholar]
- Dehydroepisterone (DHEA) supplementation in diminished ovarian reserve (DOR) Reprod Biol Endocrinol. 2011;9:67.
- [Google Scholar]
- Role of DHEA in diminished Ovarian reserve, systematic review. World J. Pharmaceut. Res.. 2015;4:2488-507.
- [Google Scholar]







