Translate this page into:
To study the comparison of efficacy of letrozole versus clomiphene citrate for ovulation induction in infertile women with PCOS in Indian population
Address for correspondence: Dr Eshna Gupta, Fellow in Clinical ART, Room no. 711, Department of Reproductive Medicine, Akanksha IVF Centre, Mata Chanan Devi Hospital, C-1, Janakpuri, New Delhi, India. E-mail: guptaeshna@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
The aim of the study was to study the comparison of the efficacy of letrozole versus clomiphene citrate (CC) for ovulation induction in infertile women with polycystic ovary syndrome (PCOS) in Indian population.
Methods:
This prospective trial included 92 infertile women with PCOS. Letrozole dose of 2.5 mg/day (n = 47) or a CC dose of 100 mg/day (n = 45) was given from day 3 to day 7 of the menstrual cycle. Follicular monitoring was started from day 7 and done until the mean diameter of the largest follicle reached 18 mm, then, 5000 IU of human chorionic gonadotropin (HCG) was given. Intrauterine insemination was done in two sittings, 24 and 48 hours after HCG. Statistical testing was conducted with SPSS 17.0.
Results:
The clinical profile of patients belonging to both groups were comparable. The number of follicles ≥18 mm on the day of HCG was 1.11 ± 0.43 for the letrozole group and 2.53 ± 1.10 for the CC group (P<0.001). Endometrial thickness on the day of HCG administration (mm) was higher in the letrozole group than the CC group (P<0.001). Ovulation rate was higher in the letrozole group (P = 0.047). Urine pregnancy test (UPT) positive patients were 40.42% in the letrozole group and 20% in the CC group (P = 0.033). Clinical pregnancy rates were slightly higher in the letrozole group. Higher twin pregnancy (P = 0.025) and miscarriage rates were noted in the CC group (P = 0.033).
Conclusion:
Letrozole appears to be superior to CC for ovulation induction in infertile PCOS patients.
Keywords
Clomiphene citrate
letrozole
PCOS
INTRODUCTION
Polycystic ovary syndrome (PCOS) is among the most common endocrine disorders in women of reproductive age, with an estimated prevalence of 5% to 10% of the general population, and by far the most common cause of anovulatory infertility.[1] PCOS group II is the predominant cause of anovulatory infertility, accounting for >80% to 90% of all cases.[2] Common signs and symptoms of PCOS include hirsutism, acne, alopecia, and irregular menstruation; furthermore, obesity is a common association.
The antiestrogens induce gonadotrophin release from the pituitary gland by occupying the estrogen receptors in the hypothalamus, thereby interfering with the normal feedback mechanisms, that is blocking the negative feedback effect of estradiol (E2).[2] This leads to increased FSH secretion, which in turn stimulates follicular growth. Both clomiphene citrate (CC) and Tamoxifen have been the first choice agents for treating anovulation since their first use in the early 1960s.[3] CC is a commonly prescribed pharmacologic agent used to induce ovulation in women with PCOS. It works as a selective estrogen receptor modulator by competitively attaching to the nuclear estrogen receptors. As the negative feedback of estrogen is reduced, secretion of gonadotropin hormones increases, inducing ovarian follicular growth. CC also has an antiestrogenic effect on endometrial development and cervical mucus production, which has been suggested to contribute to a relatively low pregnancy rate despite a high ovulation rate.[4,5] Therefore, an oral drug that can be used as a safe alternative to CC for ovulation induction was necessary.
Letrozole has been in the market as an ovulation-inducing agent. It works as a highly selective aromatase inhibitor, preventing androgen-to-estrogen conversion. One proposed mechanism is via suppressed estrogen production resulting in decreased negative feedback on the hypothalamus and increased secretion of follicle stimulating hormone (FSH). An additional proposed mechanism of improved ovulatory rates with the use of letrozole is increased follicular sensitivity to FSH resulting from temporarily increased intraovarian androgens.[6] It has a short half-life (45 hours); hence, it is rapidly eliminated from the body without producing long-lasting adverse effects on cervical mucus and endometrial thickness. Furthermore, it does not downregulate the estrogen receptors as compared to CC.[7] Letrozole has been shown to have a good ovulation rate in CC-resistant PCOS women.[8] Indian PCOS women have a high prevalence of insulin resistance[9] and thus are likely to have high CC resistance. Letrozole could prove to be a good alternative for ovulation induction in such women.
MATERIALS AND METHODS
The present study was a prospective interventional study conducted at Akanksha IVF Centre, Mata Chanan Devi Hospital, New Delhi over a period of 8 months from August 2019 to March 2020. The study was conducted in the patients of PCOS, diagnosed by Rotterdam criteria, who had previously failed to conceive or ovulate. After obtaining ethical clearance and consent, 92 patients were included in the study wherein 47 were assigned in Group 1 with letrozole stimulation (Group L) and 45 in Group 2 with CC stimulation (Group C) for intrauterine insemination (IUI) cycle.
Inclusion criteria were: women between 23 and 38 years of age, women diagnosed with PCOS according to the Rotterdam criteria, duration of infertility >1 year, at least one tubal patency confirmed on hysterosalpingography, and normal uterine cavity (as determined by sonohysterography, a combined hysteroscopy and laparoscopy, or evidence of an intrauterine pregnancy within the previous 3 years). Exclusion criteria were: women older than 38 years of age, severe endometriosis (stage IV), male factor infertility, and any other endocrinological disorder. The patients who fulfilled the inclusion criteria were enrolled in this study. Ovulation induction was started on day 2 or 3 of a spontaneous menstrual cycle or after withdrawal bleed. After doing a baseline day 2 scan and ensuring a thin endometrium thickness and absence of functional ovarian cyst, ovulation induction was started. Pretreatment endometrial thickness was taken and serum FSH, luteinizing hormone (LH), serum E2, and progesterone levels were done on day 2 or 3 of the cycle for all the patients who fulfilled the inclusion criteria. Patients were randomized using a computer-generated random table in two groups and were treated with 2.5 mg of letrozole daily (47 patients) or 100 mg of CC daily (45 patients) for 5 days from day 3 to day 7 of their menstrual cycle. Follicular monitoring was started from day 7 and done until the mean diameter of the largest follicle reached 18 mm. In addition, the endometrial thickness (mm) was measured. When the mean diameter of at least one follicle reached 18 mm, 5000 IU of human chorionic gonadotropin (HCG) was administered intramuscularly. The occurrence of ovulation, number of mature follicles (≥18 mm diameter), and endometrial thickness were measured on the day of HCG. The occurrence of ovulation was documented on transvaginal ultrasonography (TVS) by the disappearance or decrease in size of the dominant follicle and fluid in the pouch of Douglas.
IUI was done in two sittings, 24 and 48 hours after HCG. On both days, TVS was done before IUI to check for ovulation. All women received 400 mg of micronized progesterone intravaginally daily for 15 days.
Urine pregnancy test (UPT) was done after 15 days and if positive, a TVS was done at 6 weeks of pregnancy for testing clinical pregnancy. Miscarriage rates were also noted.
Statistical testing was conducted with the statistical package for the social science system version SPSS 17.0. For all statistical tests, a P-value < 0.05 was taken to indicate a significant difference.
OBSERVATIONS AND RESULTS
Patient characteristics such as age, duration of infertility, body mass index, presenting signs and symptoms, and baseline investigations were kept similar in both groups as depicted in Table 1.

Table 2 shows the laboratory parameters of the studied patients. For both groups, FSH, LH, E2, anti-mullerian hormone (AMH), total cholesterol, TSH, and prolactin levels were measured and the association was found to be statistically insignificant (P>0.05) between both groups. The ultrasonography (USG) findings were also comparable between both the groups.
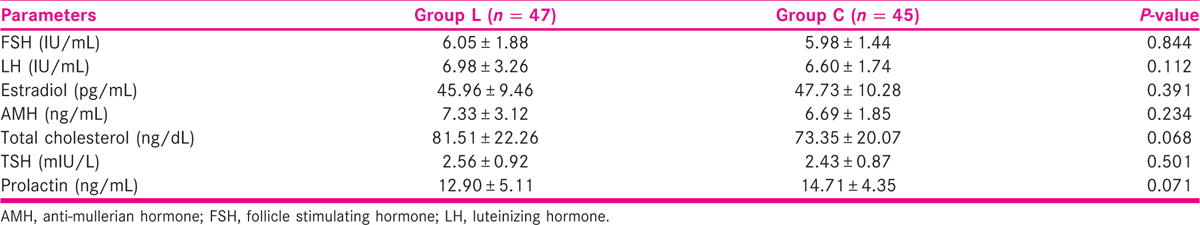
Table 3 shows the HCG details of the studied patients and depicted that on the day of HCG administration, follicular development was found to be statistically insignificant in both groups (P > 0.05) except for the number of follicles ≥18 mm and the endometrial thickness.
The number of follicles ≥18 mm on the day of HCG administration was 1.11 ± 0.43 for group L and 2.53 ± 1.10 for group C (P < 0.001), suggesting more monofollicular growth with letrozole and multifollicular growth with CC.
Also, the endometrial thickness on the day of HCG administration was 8.06 ± 0.83 mm for group L and 7.34 ± 0.94 mm for group C that was found to be statistically significant (P < 0.001) that suggests possible antiestrogenic side effects of CC on the endometrium, thus, indicating toward poor implantation rates and pregnancy losses.
Figure 1 shows the ovulation rate and urine pregnancy test positive patients in the studied groups. Ovulation rates for group L were 80.85%, whereas for group C, they were 62.2%, which was statistically significant (P = 0.047). Similarly, urine pregnancy test positive patients were 19 of 47 (40.42) in group L and nine of 45 (20%) in group C, and these values were statistically significant (P = 0.033).
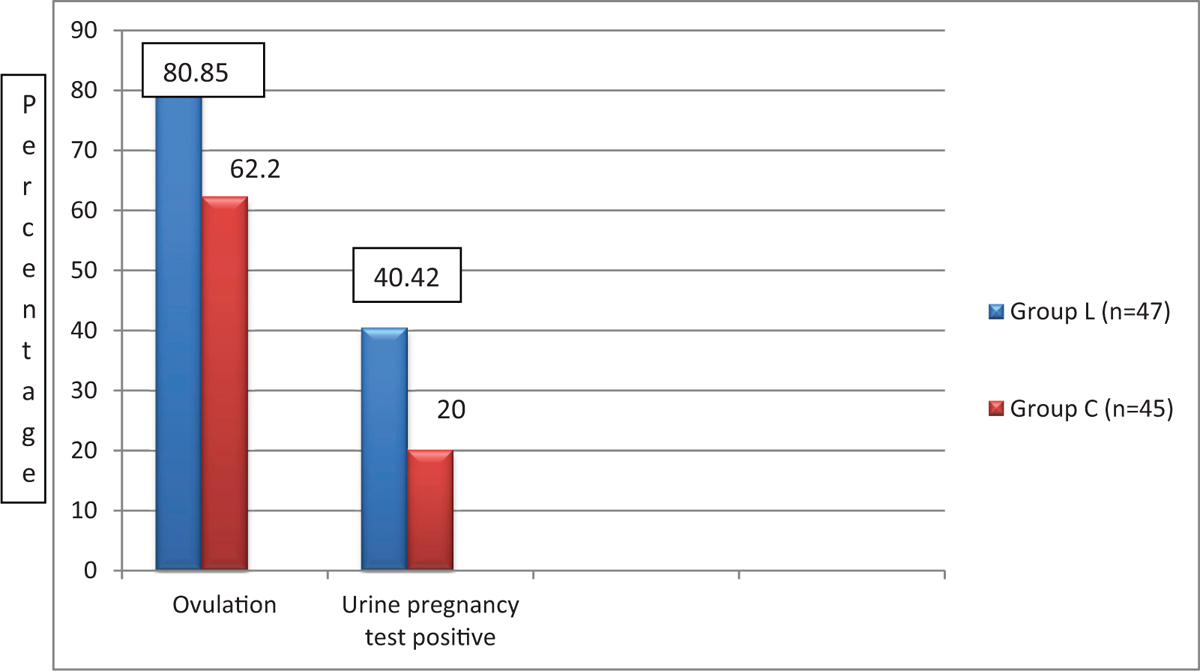
- Ovulation and urine pregnancy test positive.
Clinical pregnancy rates were slightly higher in group L (16 of 47 patients) compared to group C (eight of 45 patients) and the results were statistically insignificant (P = 0.075). Group L had no evidence of twins but in group C, five of eight patients were diagnosed with twins in early pregnancy scan; this finding was statistically significant (P = 0.025). There was only one miscarriage in group L, and in group C, it was observed that six of eight patients had a miscarriage, and this finding was also statistically significant (P = 0.033), as shown in Figure 2. One and three patients had biochemical pregnancy in the CC group and the letrozole group, respectively.
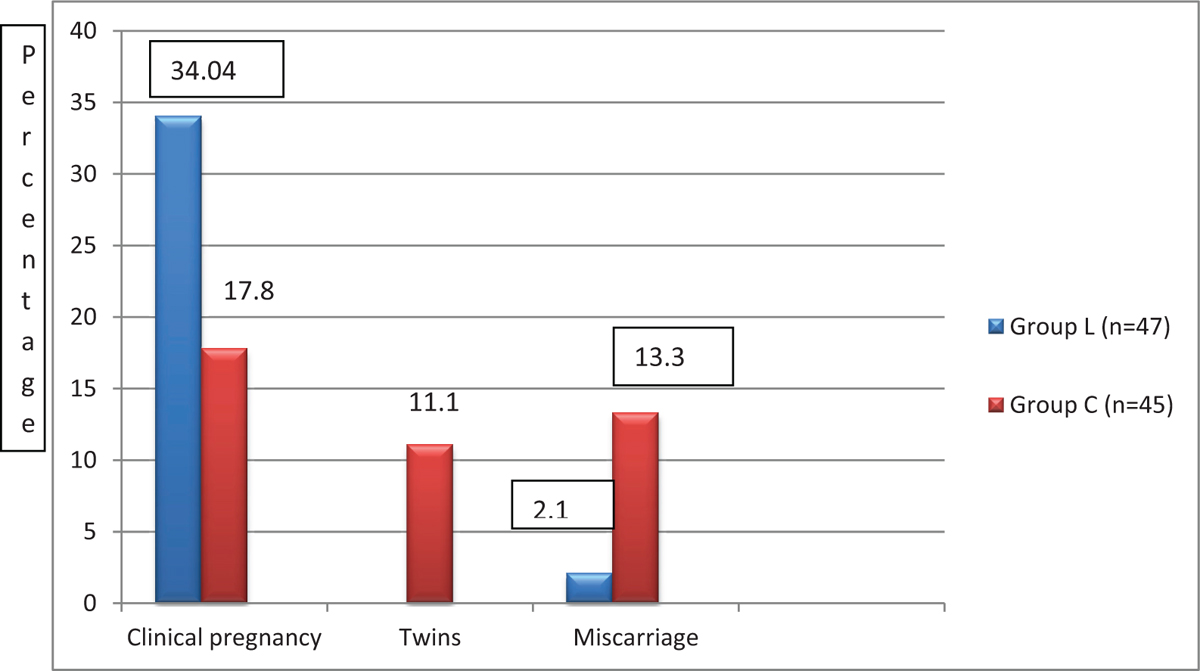
- Pregnancy outcome based on urine pregnancy test positive.
There were no significant side effects observed during the treatment in both the groups and the association was found to be statistically insignificant. In group L, only one patient had a headache, two experienced headache and abdominal pain, two had headache and hot flashes, and only one complained of nausea and abdominal pain. In group C, two patients complained of abdominal pain, two patients experienced hot flashes, one had headache and nausea, and one had hot flashes and nausea. But none of the patients in both groups required any additional hospital care for the side effects, and the regimen was acceptable to all the patients and if needed, the patients in both groups were ready to take the regimen again.
DISCUSSION
For anovulatory infertile patients with PCOS, ovulation induction is an essential treatment. It is considered as the most common endocrine disorder in women, with a prevalence of 6% to 10% based on the National Institute of Health Criteria, and as high as 15% when the broader Rotterdam criteria are applied.[10]
We found in our study that on the day of HCG administration, the number of follicles ≥18 mm was statistically significantly higher in Group C (2.53 ± 1.10) compared to Group L (1.11 ± 0.43), whereas follicular development was found to be statistically insignificant (P > 0.05) and endometrial thickness was found significantly higher in group L (8.06 ± 0.83) than group C (7.34 ± 0.94; P < 0.05). Our findings were supported by an Indian study by Hegde et al.[11] in which mean endometrial thickness was significantly higher in letrozole group, 9.18 ± 1.49, compared to CC, 7.86 ± 1.25. Similarly, Chakravorty et al.[12] reported no statistically significant difference in the pretreatment endometrial thickness between the two groups, while the endometrial thickness at the time of HCG administration was statistically significantly greater in the letrozole group (9.82 ± 0.7 vs 8.13 ± 0.56; P < 0.0001). Elsemary et al.[13] also found that letrozole was associated with greater endometrial thickness. But inconsistent results were reported by Banerjee Ray et al.[14] A total of 147 Indian women with PCOS were enrolled and divided in letrozole (2.5 mg) and CC (100 mg) groups. Mean endometrial development was 8.72 ± 11.41 mm in the letrozole group and 8.78 ± 1.16 mm in the CC group (P = 0.004). Another study was done by Badawy et al.[15] on 438 patients with 1063 cycles, one of the largest studies comparing CC and letrozole, reported statistically significantly higher endometrial thickness in the CC group (9.2 ± 0.7) versus the letrozole group (8.1 ± 0.2, P = 0.021). They attributed this effect to a greater number of mature follicles and higher serum E2 levels.
In a study by Sharief and Nafee[16] that was conducted on 75 Iraqi women, the findings showed that the number of mature follicles (18 mm follicles) was significantly lower, but the endometrial thickness and ovulation were significantly higher in the Letrazole group than in the CC group (P < 0.05 each). Women with PCOS have relatively low levels of ovarian aromatase in comparison with non-PCOS patients. High exogenous FSH or low-estrogen production due to aromatase inhibitors will lead to the growth of one or more ovarian follicles instead of multifollicular development.[14] This could be one more reason for letrozole to be a better first-line drug compared to CC.
Letrozole was found to be as effective as CC in inducing ovulation and with a slightly higher pregnancy rate as reported by Rajashekar et al.[17] Similarly, in the present study, ovulation (80.85% in group L versus 62.2% in group C) and urine pregnancy test (40.42% positive in group L versus 20% in group C) were seen and both tests were statistically higher in group L than in group C (P < 0.05). The findings of our study were in accordance with Chakravorty et al.[12] who reported that ovulation occurred in 25 subjects (37.87%) in the letrozole group and 13 subjects (19.67%) in the CC group, with a statistically significant difference between the two groups (P = 0.024). Airao et al.[18] reported ovulation rate of 60.02% in the CC group and 72.28% in the letrozole group similar to the present study.
Preliminary studies reported that aromatase inhibitors (letrozole) were useful for inducing ovulation and superovulation. Letrozole has also been shown to be effective in ovulation induction in CC-resistant PCOS women.[8] Hyper-insulinemia, which is closely associated with PCOS, is thought to be one of the causative factors for CC resistance. The prevalence of insulin resistance in PCOS is close to 50%.[19] This could be one more reason for letrozole to be a better first-line drug compared to CC. In the majority of the studies, no statistically significant difference is found between CC and letrozole in terms of ovulation rate.
In our study, the association of twins and miscarriage was found to be statistically higher in group C than in group L (P < 0.05) whereas the clinical pregnancy rate was higher in group L than group C. Our findings were similar to Airao et al.[18] who depicted the pregnancy rate of 7.42% in the CC group and 20.55% in the L group. There was one case of multiple pregnancies in group C and none in group L. Elsemary et al.[13] reported that pregnancy occurred in eight (9.7%) of 82 cycles in the CC group and six (7.6%) of 78 cycles in the letrozole group; the difference was not statistically significant. There were neither miscarriages nor multiple pregnancies in either group and the results were in contrast to our findings that may be because of sample size or the dose of the medicine. The possible reason for better pregnancy rates with letrozole in this study could be a combination of better ovulation rates and better endometrial thickness facilitating implantation of the fertilized ovum. Although the number of pregnancies in the present study is significantly higher in the letrozole group compared with the CC group, there was no significant difference in pregnancy rate per cycle. This may be due to the fact that pregnancy depends on multiple factors in a particular cycle and therefore may not reflect the difference. Letrozole has fewer side effects, a shorter half-life than CC, and has no adverse effects on endometrial receptivity. Also, its safety is superior to that of CC. Using bioequivalent doses, letrozole pregnancy rates are equal or superior to those achieved with CC.[20] In the present study, the association of the acceptability of side effects was found to be statistically insignificant (P > 0.05). Our findings were in accordance with Rachel et al.[21] who reported that there was no significant difference in the side-effect profile between the two groups. The most commonly reported side effects in the letrozole group included headache (41%), fatigue (22%), and abdominal pain or cramping (19%). The most commonly reported side effects in the letrozole and CC groups included: hot flushes (31%), headache (28%), and abdominal pain or cramping (19%).
In the present study, the association was statistically insignificant as the regimen was acceptable to the patients of both the groups and they were ready to take the regimen again (P > 0.05). Our findings were similar to Rachel et al.[21] who reported similar side-effect profiles of the two treatments. Only one participant out of 32 said they would not take the regimen again, owing to irritability.
Limitations of the study
One of the limitations of our study was the relatively small sample size.
Only one cycle per participant was performed and there was not a stair-step approach for increasing the dose in either treatment group.
CONCLUSION
Thus, letrozole is more effective in inducing ovulation in patients with anovulatory PCOS than CC in terms of monofollicular ovulation and a better endometrial thickness. The mean diameter of the largest follicle and ovulation rate showed that letrozole was superior to CC. Also, multiple pregnancies and miscarriages were low with letrozole.
Hence, letrozole can be recommended as the first-line drug for ovulation induction in anovulatory patients with PCOS.
Compliance with ethical standards
Ethical clearance from the ethical committee had been taken before proceeding with the study.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors report no conflicts of interest.
REFERENCES
- Infertility in Practice (3rd). London, UK: Informa Healthcare; 2008. http://dx.doi.org/10.3109/9781439807224.
- Outcome of pregnancy after clomiphene therapy. Acta Obstet Gynecol Scand. 1976;55:371-5. http://dx.doi.org/10.3109/00016347609158516
- [Google Scholar]
- Sonographic determination of a possible adverse effect of clomiphene citrate on endometrial growth. Hum Reprod. 1990;5:670-4.
- [Google Scholar]
- Cervical mucus score and in vitro sperm mucus interaction in spontaneous and clomiphene citrate cycles. Fertil Steril. 1991;56:465-8.
- [Google Scholar]
- Aromatase inhibitors in ovarian stimulation. J Steroid Biochem Mol Biol. 2007;106:71-5.
- [Google Scholar]
- Role of aromatase inhibitor in ovulation induction in patients with poor response to clomiphene citrate. J Obstet Gynaecol Res. 2006;32:502-6.
- [Google Scholar]
- Comparison of letrozole with continuous gonadotropins and clomiphene gonadotropin combination for ovulation induction in1387 PCOS women after clomiphene citrate failure: a randomized prospective clinical trial. J Assist Reprod Genet. 2009;26:19-24.
- [Google Scholar]
- Consensus on women’s health aspects of polycystic ovary syndrome (PCOS) Hum Reprod. 2012;27:14-24.
- [Google Scholar]
- Comparison of the role of letrozole & clomiphene citrate as a first-line ovulation induction drug in infertile women with polycystic ovary syndrome. Indian J Obstet Gynecol Res. 2020;7:12-15.
- [Google Scholar]
- A prospective, randomized trial comparing the effects of letrozole versus clomiphene citrate for induction of ovulation and pregnancy rate in women with polycystic ovary syndrome. Fertil Sci Res. 2016;3:93-97.
- [Google Scholar]
- Ovulation induction in women with polycystic ovarian syndrome: clomiphene citrate or letrozole? J Med Sci Res. 2018;1:26-29.
- [Google Scholar]
- Comparison of efficacy of letrozole and clomiphene citrate in ovulation induction in Indian women with polycystic ovarian syndrome. Arch Gynecol Obstet. 2012;285:873-7.
- [Google Scholar]
- Clomiphene citrate or letrozole for ovulation induction in women with polycystic ovarian syndrome: a prospective randomized trial. Fertil Steril. 2009;92:849-52.
- [Google Scholar]
- Comparison of letrazole and clomiphene citrate in women with polycystic ovaries undergoing ovarian stimulation. J Pak Med Assoc. 2015;65:1149-52.
- [Google Scholar]
- Polycystic ovaries and infertility: our experience. J Hum Reprod Sci. 2008;1:65-72.
- [Google Scholar]
- Comparison of the efficacy of letrozole and clomiphene citrate for ovulation induction in infertile women with polycystic ovary syndrome. Int J Curr Res. 2019;11:4346-9.
- [Google Scholar]
- Prevalence of endocrine diseases and abnormal glucose tolerance tests in 340 Caucasian premenopausal women with hirsutism as referral diagnosis. Fertil Steril. 2004;84:1570-9.
- [Google Scholar]
- Letrozole for ovulation induction and controlled ovarian hyperstimulation. Curr Opin Obstet Gynecol. 2010;22:289-94.
- [Google Scholar]
- A randomized controlled trial of combination letrozole and clomiphene citrate or letrozole alone for ovulation induction in women with polycystic ovary syndrome. Fertil Steril. 2019;111:P571-8. E1.
- [Google Scholar]







