Translate this page into:
Sperm DNA fragmentation − can it be a routine?
Address for correspondence: Dr Sasikala Natarajamani, Crea Conceptions Private Limited, No 2/220, Second Floor, Mount Poonamallee High Road, Kattupakkam, Chennai 600056, Tamil Nadu, India. E-mail: sasikala@creaconceptions.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aim:
To assess the correlation between semen parameters and sperm DNA fragmentation (SDF) and to examine whether SDF can be recommended as a routine test along with semen analysis.
Materials and methods:
Retrospective analysis was conducted for 118 male infertile patients attending a fertility clinic obtained from the records between 2017 and 2019. Semen analysis and SDF were assessed according to World Health Organization − 2010 guidelines and sperm chromatin dispersion test, respectively. Patients were grouped based on their SDF scores as “low DNA fragmentation group (LDF)” if SDF ≤ 18% and as “high DNA fragmentation group (HDF)” if SDF > 18%. Mann–Whitney and Kruskal–Wallis tests were used to find the difference in the mean of semen parameters between SDF groups. Statistical analysis was carried out using STATA Version-14.
Results:
The mean value of sperm concentration, motility, and normal morphology was higher in LDF compared to HDF. Correlation analysis showed patients’ age to be positively associated with SDF (r = 0.125; P = 0.102). Sperm concentration, progressive motility, and normal morphology had a weak negative correlation with SDF. Around 63% and 69% of patients with normal morphology and normal motility respectively had high SDF. In the HDF category, nearly 42% had normal morphology, whereas a 66% demonstrated normal motility. The results of Kruskal–Wallis test for patients who had a treatment outcome (conception) showed that patients in LDF group had 1.5 times higher chance of “conception and live birth.”
Conclusion:
Men with normal semen parameters can still have a high level of SDF. SDF along with semen analysis can help assess the reproductive potential of a male better.
Keywords
Andrology
assisted reproduction technologies
male infertility
semen analysis
sperm DNA fragmentation
INTRODUCTION
Male infertility is on the rise and among couples attending fertility clinics for treatment, almost 50% are due to male factor.[1,2,3] To diagnose male infertility, traditionally, semen analysis is being performed as the primary investigation. Several parameters are assessed during a routine semen analysis including the volume, appearance, pH, count, motility, morphology, and leukocyte concentration according to the guidelines issued by World Health Organization (WHO).[4] In addition to semen analysis, physical examination, hormonal analysis, and Doppler imaging are the other tests used to assess male infertility. Although semen analysis reveals useful information at a basic level, it is not a complete test of the fertility of an individual.[5,6] It provides no insights into the functional potential of the spermatozoon to undergo subsequent maturation process required to achieve fertilization.[7] Though the results may correlate with “fertility,” in general, the assay itself is not a direct measure of fertility.[8,9,10]
In recent years, there is emerging evidence that sperm deoxyribonucleic acid (DNA) integrity plays an important role in determining the outcome of an assisted conception cycle.[11,12,13,14] Chromatin, in somatic cells, is a relatively loose structure, whereas the chromatin in sperm cells is very tightly compacted because of the small head size.[15] Damage to sperm DNA may occur as a result of intrinsic factors such as defects in maturation process, oxidative stress, and abortive apoptosis or as a result of extrinsic factors such as medications, lifestyle choices, and environment, some of which is repaired by the oocyte.[16,17] Oxidative DNA damage is an important factor that affects sperm quality and increases risk of genetic and epigenetic abnormalities.[18] High sperm DNA fragmentation (SDF) is known to affect fertilization rates, early embryo development, implantation rates, and clinical pregnancy rates.[19,20,21] High SDF rates are also proven to be associated with recurrent spontaneous abortions and might influence the outcome of assisted conception cycles.[22,23,24]
Due to its significant role in fertilization and further embryo development, SDF has the potential to become a routine test in addition to semen analysis during the treatment of infertile couples. But is it necessary to perform both as a routine or either one is enough to predict the fertilization potential of an individual? How do they correlate with each other? In an effort to understand this, we tried to correlate semen parameters such as age, volume, sperm count, sperm motility, sperm morphology, and leukocytospermia with the SDF of semen samples and their overall impact on the outcome of an assisted reproductive technology (ART) cycles.
MATERIALS AND METHODS
The records of 118 infertile couples from a tertiary ART center from 2017 to 2019 were selected retrospectively for analysis. Patients with only male factor infertility were included and those with azoospermia and sperm concentration ≤5 million/mL were excluded. Semen analysis was performed according to WHO standards 2010[4] and SDF index (DFI) was determined by sperm chromatin dispersion (SCD) assay.
Semen analysis
Following 4 days of abstinence, a complete sample of neat ejaculate was collected in the laboratory premises by masturbation into a sterile container. The sample was allowed to liquefy for 45 minutes to an hour. Sperm concentration was calculated using the Makler chamber. Sperm motility was assessed using a wet slide examined with phase-contrast optics at ×400 magnification. An air-dried, fixed, and stained preparation was made for sperm morphology determination by using bright-field optics. The semen parameters analyzed in this study were sperm concentration, motility, and morphology.[4]
Sperm DNA fragmentation
The percentage of SDF was determined by SCD assay using Halo sperm® kit (Halotech DNA SL, Madrid).[25] SCD is based on the principle that mild acid denaturation of DNA followed by lysis of protamine’s creates a chromatin decondensation halo around sperm head when DNA is intact and no halo when DNA is damaged. Briefly, 25 μL of semen sample was diluted with culture medium to produce a concentration of 5 to 10 million/mL sperm cells. The sample was then embedded in agarose on a pretreated slide, incubated with acid denaturation solution followed by a lysis solution. The slides were then exposed to dehydrants (70%, 90%, and 100% ethanol, respectively) and stained using Diff-Quik staining technique. The stained slides were air dried and visualized under a standard bright field microscope. Sperms with intact DNA appear with big- or medium-sized halo, whereas those with fragmented DNA appear with small or no halo. Five hundred sperm cells were analyzed for each semen sample and the percentage of SDF was calculated.
Statistical analysis
We grouped the values into two based on percentage of SDF, a low fragmentation DNA group (LDF) with SDF ≤ 18% and high DNA fragmentation group (HDF) with SDF > 18%.[26] The statistical analysis was performed using STATA Version-14 (Stata Corp. LLC, Texas, USA). The degree of relationship between the SDF and semen parameters was estimated by bivariate Pearson correlation coefficients. Mann–Whitney tests were used to compare the parameters between HDF and LDF. Kruskal–Wallis test was used to find the association between the semen parameters and the outcome of the study grouped as for 66 patients.
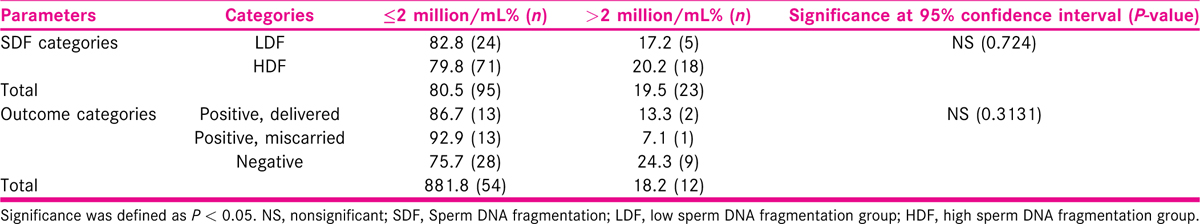
Understanding the nonparametric nature of the data, we have also conducted Mann–Whitney tests when comparing the parameters between HDF and LDF. The statistical significance was measured at the 95% confidence interval (P < 0.05). We analyzed 66 patients out of the total 118 samples which received an outcome at the end of the treatment process. The outcome was categorized as “positive, delivered” (n = 15), “positive, miscarried” (n = 14), and “negative” (n = 37). Kruskal–Wallis test was conducted to understand the significance of the distribution of semen parameters across the outcome categories. We also categorized SDF groups and outcomes based on the number of leukocytes: ≤2 million/mL and >2 million/mL.
RESULTS
For the overall sample, the mean sperm concentration was 64.34 (±39.79) × 106/mL, and the mean estimate was much higher in LDF when compared with HDF. Mean volume of the sample was 2.45 mL, and the mean volume was 0.27 mL higher in LDF when compared with HDF. Mean percentages of progressive motility were 66.48% (±8.45%) and 54.62% (±16.96%) for LDF and HDF, respectively. Mean morphologically normal sperm was 5.14 ± 2.84% for LDF and 3.48 ± 2.0% for HDF. Even though the mean percentages of sperms with head defect for LDF and HDF do not show statistical significance, we observed a significant mean difference between these two SDF groups in terms of percentage of sperms with multiple defects [Table 1]. At 95% confidence interval, there is no statistically significant association between SDF and age of the patient [Figure 1]. The correlation between SDF and sperm concentration shows that there is a statistically significant negative association (r = −0.183), which means that when sperm concentration increases, SDF declines [Figure 2]. On the contrary, percentage of SDF increases with higher percentage of immotile sperms (r = 0.395) and vice versa [Figure 3]. Sperms with progressive motility demonstrate a statistically significant and negative association with SDF (r = −0.381), as depicted in Figure 4. According to Figure 5, there is a statistically significant negative association between normal morphology and SDF, that is, as the percentage of sperms with normal morphology increases in a sample, SDF percentage decreases (r = −0.318). Interestingly, the significant correlation coefficient between SDF and percentage of sperms with head defects in a sample shows that they are inversely correlated with each other [Figure 6]. The association with multiple defect morphology has been found to be statistically significant and positive, which means that the percentage of SDF increases with the increase in percentage of sperms with multiple defects morphology (r = 0.274) [Figure 7].

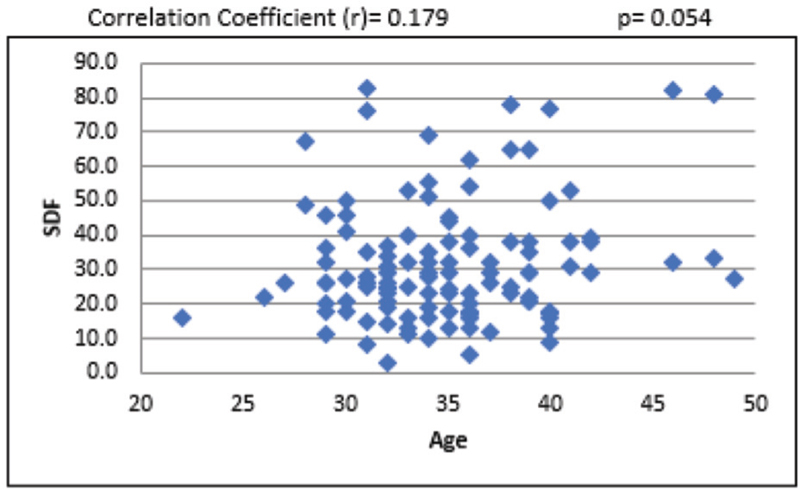
- Scattered diagram of age of patients with SDF. SDF, sperm DNA fragmentation.

- Scattered diagram of sperm concentration with SDF. SDF, sperm DNA fragmentation.
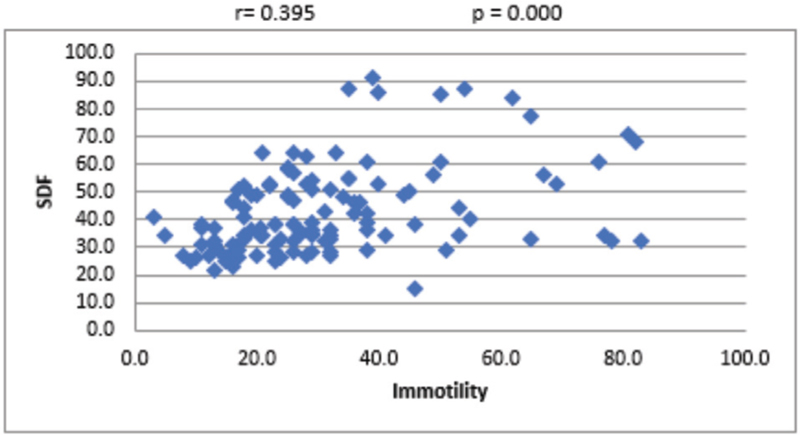
- Scattered diagram of sperm immotility (%) with SDF. SDF, sperm DNA fragmentation.
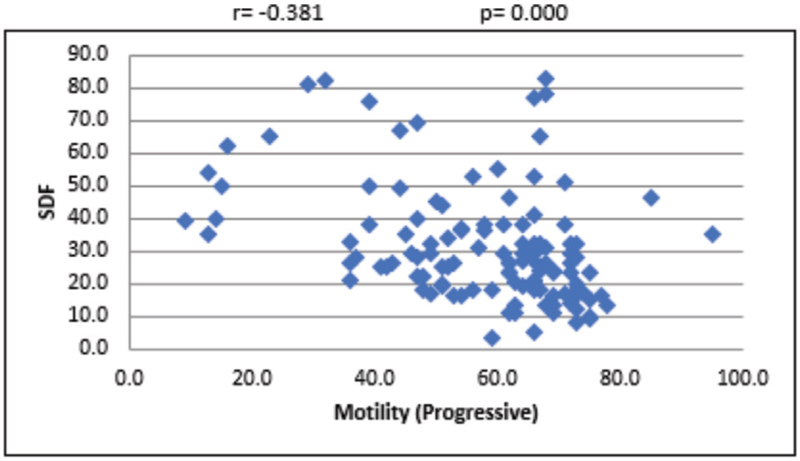
- Scattered diagram of progressive motility (%) with SDF. SDF, sperm DNA fragmentation.
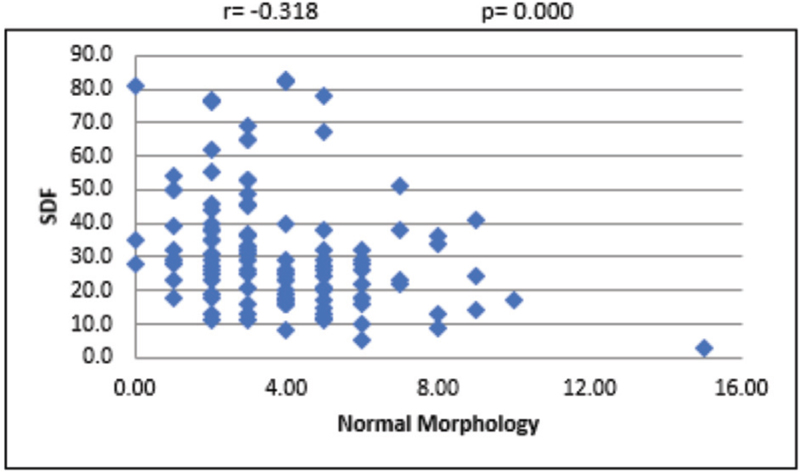
- Scattered diagram of normal morphology sperm (%) with SDF. SDF, sperm DNA fragmentation.
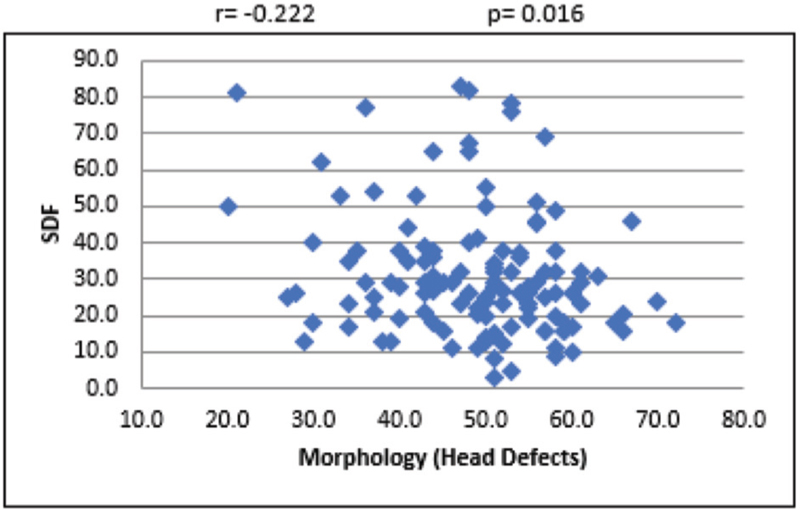
- Scattered diagram of head defect morphology sperm (%) with SDF. SDF, sperm DNA fragmentation.
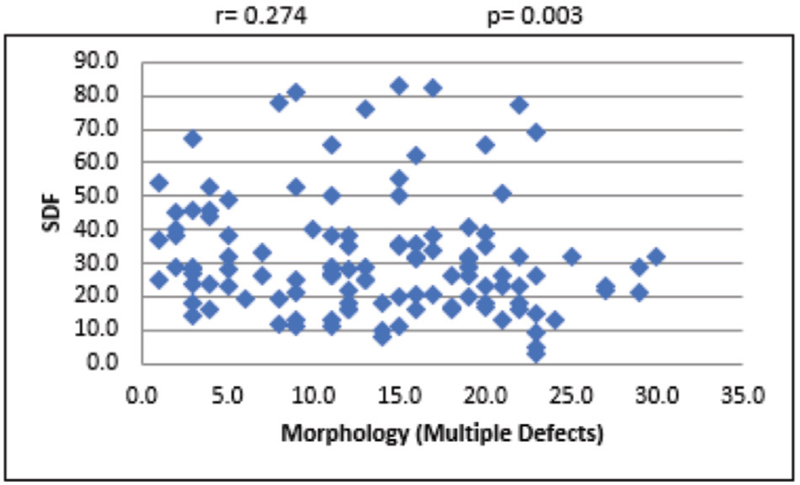
- Scattered diagram of multiple defect morphology sperm (%) with SDF. SDF, sperm DNA fragmentation.
Table 2 demonstrates the results of descriptive statistics across the treatment outcome categories and their statistical significance based on the Kruskal–Wallis test. Apart from fast progressive motility and normal sperm morphology, mean differences were not statistically significant for any other semen parameter while deciphering the treatment outcomes, as shown in Figures 8 and 9.

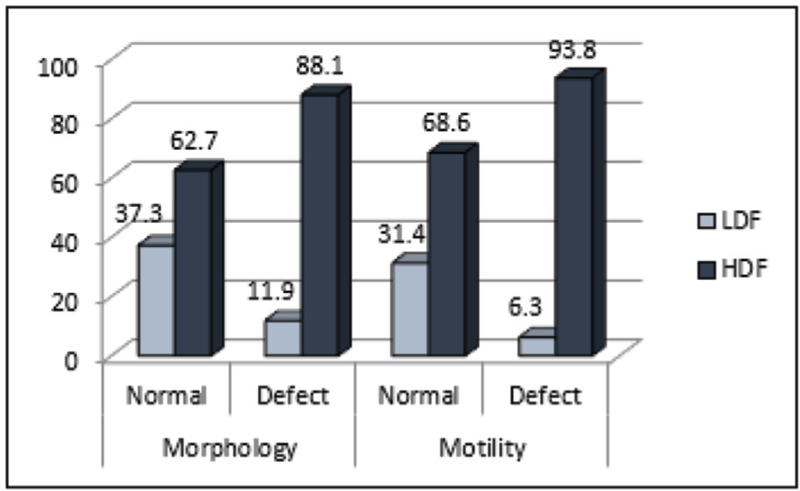
- Percentage distribution of sperm DNA fragmentation categories across motility and morphology status. HDF, high sperm DNA fragmentation group; LDF, low sperm DNA fragmentation group.
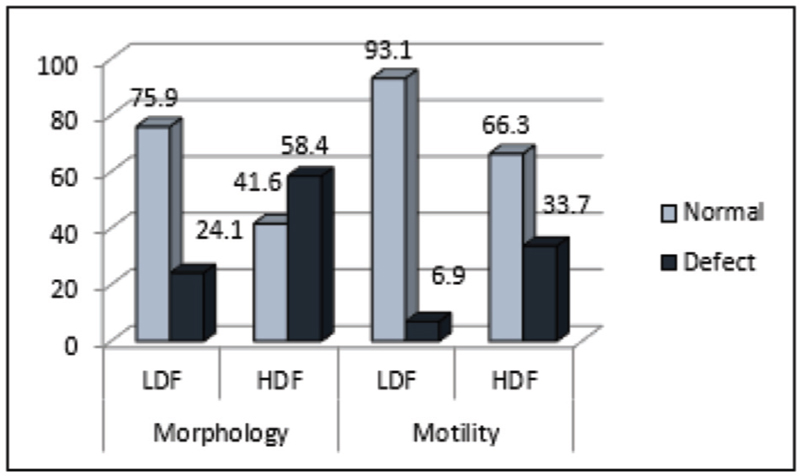
- Percentage distribution of motility and morphology status among patients with high and low DNA fragmentation.
Mean age has been lower for those who could deliver a live birth, when compared with the other two categories. Mean percentages of fast progressive motility (47.53 ± 11.58) and normal morphology (5.00 ± 1.93) are also found to be higher among the couples with positive outcome and delivering a baby. Table 3 summarizes the percentage of leukocytes found in cohorts among SDF category and outcome category.
DISCUSSION
Semen analysis still remains the conventional measure for assessment of male infertility in ART practice. In recent times, it has been proven beyond doubt that SDF is an important contributing factor for early embryo development and treatment outcomes.[19,20,21] Assessment of semen parameters alone could prove to be ineffective in prediction of the reproductive outcome because there are studies that have shown that approximately 15% of infertile men may have a normal spermiogram.[27,28] On the contrary, few studies have highlighted the possibility of spontaneous pregnancy with abnormal semen parameters.[29]
During fertilization, the sperm delivers the haploid paternal genome to the oocyte and for this purpose, an intact and complete genetic material is required.[30] A human sperm cell is different from other cells in the body. The smaller size of the sperm is at the expense of the cytoplasm or cell mass. The reduced cell mass contributes to impaired production of enzymes required for genetic repair. Unlike the chromatin in a somatic cell which is a relatively loose structure, the chromatin in sperm cells very tightly compacted because of its small size. The haploid genome must adapt to a volume 40 times less than a somatic cell.[31]
The fundamental packaging unit of sperm chromatin is a toroid which has 50 to 60 kb of DNA. An individual toroid represents a DNA loop domain which is highly condensed by protamines and fixed at the nuclear matrix. Toroids are cross linked and further compacted by disulfide bonds.[31] Sperm nucleus undergoes remodelling and condensation during later stages of spermatogenesis, where the histones are replaced by transition proteins and later by protamines. The protamines are much smaller than histone and are more basic. The protamine molecule wraps the DNA strand tightly forming a loop. Compaction and stabilization of sperm nuclei is by cross linkage between the protamines formed by disulfide bonds. The sperm genome is protected from external stress by this nuclear compaction. When there is deficient packaging, the DNA strands are susceptible to damage leading to SDF.[32,33]
In the female reproductive tract, the sperm penetrates the oocyte membrane with a highly condensed nucleus. The fusion of sperm and oocyte membrane leads to decondensation and remodeling of the parental genome and the protamines are replaced with histones of the oocyte completing the fertilization process.[34]
The SDF occurs due to intrinsic factors such as structural or biochemical defects in chromatin packaging during spermatogenesis, abnormal spermatid maturation, abortive apoptosis, and oxidative stress and extrinsic factors such as time lapse from ejaculation, storage temperature and cryopreservation, varicocele, bacterial infection, advanced age, long abstinence, reaction to medicine, and exposure to environmental chemicals.[17]
As there are various assays available to measure sperm DNA damage, selection of a precise and simple method is very essential. The sperm chromatin structure assay (SCSA®), which is considered as the gold standard technique to detect SDF, measures the intensity of acridine orange fluorescence using flow cytometry.[35] Though SCSA is a robust test, not all laboratories have access to a flow cytometer or the technical expertise to perform this assay. Other assessment methods include TdT-mediated-dUTP nick end labeling (TUNEL) assay and single-cell gel electrophoresis (COMET) assay which measure both single- and double-stranded DNA breaks. These tests too need specialized equipment and expertise. In comparison, SCD assay is simple, more sensitive, and cost-effective, requires minimal laboratory equipment, and produces results comparable to SCSA.[36,37,38] In our study, we have, therefore, used SCD assay to measure the percentage of SDF.
Though several studies have compared semen parameters with SDF, their relationship is still unclear. Few studies show good positive or negative correlation between SDF and certain semen parameters.[39,40] On the contrary, there are studies which could not find any association between the two.[41,42] Hence, we embarked on the study to see how various semen parameters correlate with SDF and whether it translates into positive or negative outcome in an ART cycle. In our study, we found that volume of the ejaculate did not differ between the LDF and HDF. We also found that those group of patients having an accepted range of DNA damage (<15%) were younger [mean: 33.317, standard deviation (SD): 1.2] than those patients whose sperm DNA damage was beyond the threshold (mean: 35.583, SD: 5.8). In addition, age did have a positive role in giving positive outcomes, that is, those couples who delivered a healthy baby were young.
In our study, sperm concentration negatively correlated with SDF. Abnormal spermatid maturation could be one of the reasons for low sperm number and high SDF.[17] We found sperm motility to be negatively associated with SDF, which might translate into the fact that, those sperms which have normal motility and can swim up during semen preparation, may have low SDF.[43] Comparing the SDF index in the processed semen sample may give some detailed insight into this claim.
Boushaba and Belaaloui, in their study, found no correlation between sperm morphology and SDF but Varghese et al., in a study on Indian population, found a significantly inverse correlation of SDF with normal morphology.[26,44] Similarly, Cohen-Bacrie et al. reported that the sperm morphology and motility were inversely proportional to the DNA damage.[45] In our study, we found that the SDF was high even in sperms with good morphology. This is an important finding signifying the importance of DNA damage assessment irrespective of the morphology evaluation during semen analysis. Though assessing the sperm morphology is an important criterion for an ART procedure, the fertilizing potential is not completely assessed with morphology alone. It is reported that even a morphologically normal sperm might not have good genetic integrity.[46] Similar to a recent study, we found that there was no statistical significance between leukocyte concentration and SDF.[47]
The conflicting studies on the correlation between SDF and semen parameters may be due to the fact that numerous technical errors and biologic variations can happen during the assessment of a semen analysis leading to difficulties in clinical interpretation. The technical variations arise from a large degree of inter- and intralaboratory variability of the semen parameters.[48] Though SDF tests have lower biologic variability when compared with semen parameters, they are still easily influenced by environmental factors.[49] This brings about the need to standardize the SDF assay protocol. In this study, we have used a kit-based protocol which used the principle of SCD, as the reproducibility of halo sperm kits have been assessed earlier.[25]
Effects of sperm DNA damage is found at various levels including fertilization and postfertilization development of an embryo both in vivo and in vitro.[28,50] Though oocytes and early embryos have shown to repair sperm DNA damage, excessive damage, or improper repair by the oocyte may lead to implantation failure, early miscarriages, or diseases in the offspring.[11,17] The biologic effect of abnormal sperm chromatin structure is the combined effect of sperm chromatin damage and the capacity of the oocyte to repair the damage.[14]
It is logical to assume that a normal sperm, in terms of conventional parameters, will have an intact DNA. But how far can this be true? Do all semen samples with good motility and good morphology have intact DNA? We found in our study that 60% of samples with agreeable motility and morphology parameters, according to WHO standards, still had a high degree of SDF according to the chosen cutoff values. However, head defects were found to be inversely and significantly associated with SDF.
Though in our study, we found strong association between progressive motility, normal morphology, and SDF, in our opinion neither of the tests is sufficient by itself. In situations such as timed intercourse or intrauterine insemination (IUI), where the functional integrity of the tail is important − SDF testing alone may not suffice. Similarly, in situations where the semen sample is used for in vitro fertilization/intracytoplasmic sperm injection, semen analysis alone is insufficient, as the outcome of the treatment is very much dependent on the DNA integrity of the sperms. This may, to some extent, be corrected or repaired by younger oocyte but still knowledge of the DNA status is very important in assessing the outcome of the treatment and counseling the patients accordingly.[51]
The importance of assessing DNA status also lies in the fact that a simple regimen of antioxidants or lifestyle modifications given to the patients with high SDF prior to treatment strategies could go a long way in improving the results.[52,53] This way the patients are given a chance to save effort, finance, and time. In addition, to enrich the diagnostic value of such fundamental form of assessment, where the pre- and post-test values are compared, a parameter with less variability is required and DNA fragmentation indices may be useful due to minimal technical errors (<5%).[54]
CONCLUSION
A semen sample with normal parameters such as good progressive motility and normal morphology can still have a high SDF. Similarly, a sample with good SDF can still have abnormalities in mid-piece or tail factors which are important when modalities such as IUI or timed intercourse are considered before the patient embarks on expensive ART treatments. Hence, to assess the fertilizing capacity of the semen sample and to devise proper treatment regimens for an infertile couple, both tests may be recommended as they complement each other and will help in achieving better treatment outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements:
The author expresses her appreciation to Dr Parameaswari, the biostatistician, for her valuable support rendered for this research work. Her willingness to give her time so generously has been very much appreciated. The author also thanks the embryologists Ms Mahalakshmi and Ms Archana Manikere of Crea Conceptions for performing the semen analysis, sperm DNA fragmentation assay, and reporting the data accurately.
REFERENCES
- Research trends and perspectives of male infertility: a bibliometric analysis of 20 years of scientific literature. Andrology. 2016;4:990-1001.
- [Google Scholar]
- Characterizing semen abnormality male infertility using non-targeted blood plasma metabolomics. PLoS One. 2019;14:e0219179.
- [Google Scholar]
- Infertility in the light of new scientific reports − focus on male factor. Ann Agric Environ Med. 2016;23:227-30.
- [Google Scholar]
- WHO Laboratory Manual for the Examination and Processing of Human Semen (5th). Geneva: WHO Press; 2010.
- Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol. 2014;40:443-53.
- [Google Scholar]
- Semen analysis: a new manual and its application to the understanding of semen and its pathology. Asian J Androl. 2010;12:11-3.
- [Google Scholar]
- Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102:1502-7.
- [Google Scholar]
- Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388-93.
- [Google Scholar]
- Semen quality and sperm functional parameters in fertile Indian men. Andrologia. 2006;38:20-5.
- [Google Scholar]
- Mechanisms and clinical correlates of sperm DNA damage. Asian J Androl. 2012;14:24-31.
- [Google Scholar]
- Predictive value of sperm deoxyribonucleic acid (DNA) fragmentation index in male infertility. Basic Clin Androl. 2017;27:1.
- [Google Scholar]
- Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9:331-45.
- [Google Scholar]
- Recent knowledge concerning mammalian sperm chromatin organization and its potential weaknesses when facing oxidative challenge. Basic Clin Androl. 2014;24:6.
- [Google Scholar]
- Sperm chromatin integrity: etiologies and mechanisms of abnormality, assays, clinical importance, preventing and repairing damage. Avicenna J Med Biotechnol. 2009;1:147-60.
- [Google Scholar]
- Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci. 2012;13:14026-52.
- [Google Scholar]
- Oxidative stress induced damage to paternal genome and impact of meditation and yoga − can it reduce incidence of childhood cancer? Asian Pac J Cancer Prev. 2016;17:4517-25.
- [Google Scholar]
- Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014;29:2402-12.
- [Google Scholar]
- Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum Reprod. 2000;15:1717-22.
- [Google Scholar]
- DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005;322:33-41.
- [Google Scholar]
- Semen parameters and sperm DNA fragmentation as causes of recurrent pregnancy loss. Urology. 2011;78:792-6.
- [Google Scholar]
- The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27:2908-17.
- [Google Scholar]
- Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil Steril. 2016;105:58-64.
- [Google Scholar]
- Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84:833-42.
- [Google Scholar]
- Sperm DNA fragmentation and standard semen parameters in Algerian infertile male partners. World J Mens Health. 2015;33:1-7.
- [Google Scholar]
- Unexplained Male infertility: diagnosis and management. Int Braz J Urol. 2012;38:576-94.
- [Google Scholar]
- Sperm DNA integrity assays: diagnostic and prognostic challenges and implications in management of infertility. J Assist Reprod Genet. 2011;28:1073-85.
- [Google Scholar]
- Male fertility and reduction in semen parameters: a single tertiary-care center experience. Int J Endocrinol. 2012;2012:649149.
- [Google Scholar]
- Paternal factors contributing to embryo quality. J Assist Reprod Genet. 2018;35:1953-68.
- [Google Scholar]
- A Clinician’s Guide to Sperm DNA and Chromatin Damage. Switzerland: Springer International Publishing; 2018.
- Sperm DNA damage: clinical significance in the era of assisted reproduction. CMAJ. 2006;175:495-500.
- [Google Scholar]
- Function of sperm chromatin structural elements in fertilization and development. Mol Hum Reprod. 2010;16:30-6.
- [Google Scholar]
- In vivo and in vitro decondensation of human sperm and assisted reproduction technologies. In Vivo. 2005;19:623-30.
- [Google Scholar]
- The Sperm Chromatin Structure Assay (SCSA(®)) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim Reprod Sci. 2016;169:56-75.
- [Google Scholar]
- Measurement of sperm DNA fragmentation using bright-field microscopy: comparison between sperm chromatin dispersion test and terminal uridine nick-end labeling assay. Fertil Steril. 2010;94:1027-32.
- [Google Scholar]
- The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59-66.
- [Google Scholar]
- Comprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral COMET assay. Andrology. 2013;1:715-22.
- [Google Scholar]
- Sperm nuclear DNA fragmentation and its association with semen quality in Greek men. Andrologia. 2015;47:1166-74.
- [Google Scholar]
- Analysis of the correlation between sperm DNA integrity and conventional semen parameters in infertile men. Turk J Urol. 2015;41:191-7.
- [Google Scholar]
- Sperm nuclear DNA in ejaculates of fertile and infertile men: correlation with semen parameters. Urol J Summer. 2006;3:154-9.
- [Google Scholar]
- The influence of cigarette smoking on human sperm quality and DNA fragmentation. Toxicology. 2006;223:54-60.
- [Google Scholar]
- Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J Assist Reprod Genet. 2012;29:557-63.
- [Google Scholar]
- Human sperm DNA integrity in normal and abnormal semen samples and its correlation with sperm characteristics. Andrologia. 2009;41:207-15.
- [Google Scholar]
- Correlation between DNA damage and sperm parameters: a prospective study of 1,633 patients. Fertil Steril. 2009;91:1801-5.
- [Google Scholar]
- Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93:1027-36.
- [Google Scholar]
- The association of seminal leucocytes, interleukin-6 and interleukin-8 with sperm DNA fragmentation: a prospective study. Andrologia. 2019;51:e13428.
- [Google Scholar]
- Uncertainty of measurement and clinical value of semen analysis: has standardisation through professional guidelines helped or hindered progress? Andrology. 2016;4:763-70.
- [Google Scholar]
- Clinical correlates of the biological variation of sperm DNA fragmentation in infertile men attending an andrology outpatient clinic. Int J Androl. 2007;30:48-55.
- [Google Scholar]
- Effects of sperm chromatin integrity on fertilization rate and embryo quality following intracytoplasmic sperm injection. Avicenna J Med Biotechnol. 2009;1:173-80.
- [Google Scholar]
- To what extent can oocytes repair sperm DNA fragmentation? Fertil Steril. 2014;102:e61.
- [Google Scholar]
- Antioxidants and sperm DNA damage: a clinical perspective. J Assist Reprod Genet. 2009;26:427-32.
- [Google Scholar]
- What should be done for men with sperm DNA fragmentation? Clin Exp Reprod Med. 2018;45:101-9.
- [Google Scholar]
- Sperm DNA fragmentation: threshold value in male fertility. Hum Reprod. 2005;20:3446-51.
- [Google Scholar]








