Translate this page into:
A successful outcome in a case of nonobstructive azoospermia with repeat surgical sperm retrieval (SSR)-testicular sperm aspiration followed by microsurgical testicular sperm extraction
Address for correspondence: Dr Meenu Vashisht Ahuja, Birla Fertility and IVF, sector 8, Rohini. E-mail: meenuahuja@live.in
-
Received: ,
Accepted: ,
How to cite this article: Ahuja MV, Talwar P. A successful outcome in a case of nonobstructive azoospermia with repeat surgical sperm retrieval (SSR)-testicular sperm aspiration followed by microsurgical testicular sperm extraction. Fertil Sci Res 2023;10:219-22.
Abstract
Azoospermia is a major cause of male infertility, either obstructive or nonobstructive azoospermia (NOA). However, such couples should be offered surgical sperm retrieval to enable them to become parents with their own gametes. Cases of NOA will have higher retrieval rate with microsurgical testicular sperm extraction (micro-TESE), even though, in some cases, it may be a repeat procedure. Presenting here a case of successful twin gestation after intracytoplasmic sperm injection with sperms retrieved successfully by micro-TESE after a previous failed frozen embryo transfer with a very poor retrieval by a testicular sperm aspiration in a case of NOA.
Keywords
Azoospermia
Nonobstructive azoospermia
Testicular sperm aspiration
Microsurgical testicular sperm extraction
Intracytoplasmic sperm injection
INTRODUCTION
The male factor alone or in combination with other factors contributes to 50% infertility cases. If the factors affecting male fertility can be treated through proper andrological evaluation and intervention, it can result in positive outcomes. It is critical to conduct a complete evaluation, which includes a detailed medical history, information on past treatment, physical examination, and a minimum of two semen analyses. Additionally, sperm function tests, ultrasound study, hormonal assessment, and genetic analysis may be deemed necessary depending on the clinical and semen analysis findings.
Azoospermia is defined as the complete absence of sperm in the semen after centrifugation in at least two samples. It is observed in 1% of the general population and in 10 to 15% of infertile men. It is subdivided into two categories: obstructive azoospermia (OA) and nonobstructive azoospermia (NOA).[1]
NOA represents the most severe form of male factor infertility found in 60% cases of azoospermia.[2,3] It is a heterogeneous group with impaired spermatogenesis ranging from hypospermatogenesis and maturation arrest to Sertoli cell-only syndrome.[4,5,6]
In cases of NOA, there are isolated islands of active spermatogenesis; hence, surgically retrieved sperm by means of testicular sperm aspiration (TESA) or conventional testicular sperm extraction (C-TESE) or micro-TESE can be used to give pregnancies by means of intracytoplasmic sperm injection (ICSI).[7,8,9,10,11]
Case History
Informed consent was taken from the couple for all procedures, as the case deemed it at various steps.
Mrs. X/32 years and Mr. Y/32 years married for 5 years approached for infertility treatment in the year 2022.
Medical History and Investigations
The wife had regular periods with Anti Mullerian Hormone (AMH) of 3.74 ng/mL.
[Normal range is 1.5–4.0 ng/mL. Method of testing is enzyme-linked immunosorbent assay (ELISA).]
The husband had undergone bilateral vasoepididymal anastomosis surgery elsewhere in 2021 for suspected OA, which however failed, and azoospermia persisted.
Examination by the urologist suggested normal epididymis and testes bilaterally and thickened spermatic cords bilaterally.
Hormonal profile was as follows: testosterone 489 ng/mL, Luteinizing Hormone (LH) 9.64 mIU/mL, Follicle Stimulating Hormone (FSH) 4.48 mIU/L.
(Units of measurement mIU/mL. Normal range in adult males is 1.5–12.4 mIU/mL and for LH is 1.7–8.6 mIU/mL. Methodology of testing is ELISA.)
Treatment Journey
The patient underwent controlled ovarian stimulation using an antagonist protocol and ovum pick-up, resulted in retrieval of 20 oocytes of which 16 were metaphase 2 oocytes (M2)
On the same day, TESA was carried out for the husband. TESA is technically a simple surgery. The available surgeon was a relatively young surgeon with moderate experience in surgical sperm retrieval procedures. The right testes yielded six sperms, and the left testes yielded none.
ICSI was done for 6 oocytes, and the rest 12 oocytes were cryopreserved. This resulted in the formation of 4-day three embryos.
Frozen embryo transfer (FET) was done in October 2022—two day four embryos were transferred with grading as follows: 1* Grade 1 (good compaction), 1* Grade 2 (partial compaction). The classification of embryo grading used was as per Istanbul Consensus workshop 2010. The luteal phase support given is as follows: Tab Estrabet/estradiol 2 mg thrice a day, tab aspirin (Ecosprin) 75 mg, tab dydrogesterone (Duphaston) 10 mg twice a day and Aqsusten injection 50 mg intramuscularly daily. However, the result was negative.
After considering various options to improve sperm quality before a subsequent sperm retrieval, Inj. Hucog HP 2000IU (Human chronic gonadotropin, Bharat Serums and Vaccines Ltd. india)-2000 HP (Bharat Serum and Vaccines, India). The route of administration was subcutaneous, thrice a week for 1 month, and antioxidants had been given for the last 6 months as well.
Thereafter, he underwent microsurgical testicular sperm extraction (micro-TESE) in April 2023 by a senior urologist surgeon with significant experience in micro-TESE, which resulted in good retrieval of sperms.
Using these sperms, ICSI was done for the remaining 12 M2 oocytes and resulted in 1 day five and 3-day three embryos. (Figures 1-3)
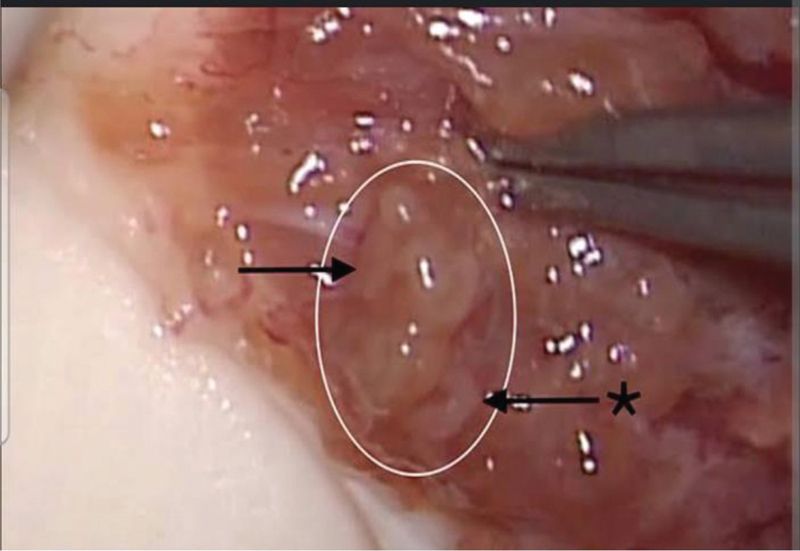
- Seminiferous tubules seen during the microsurgical testicular sperm extraction (micro-TESE) procedure.
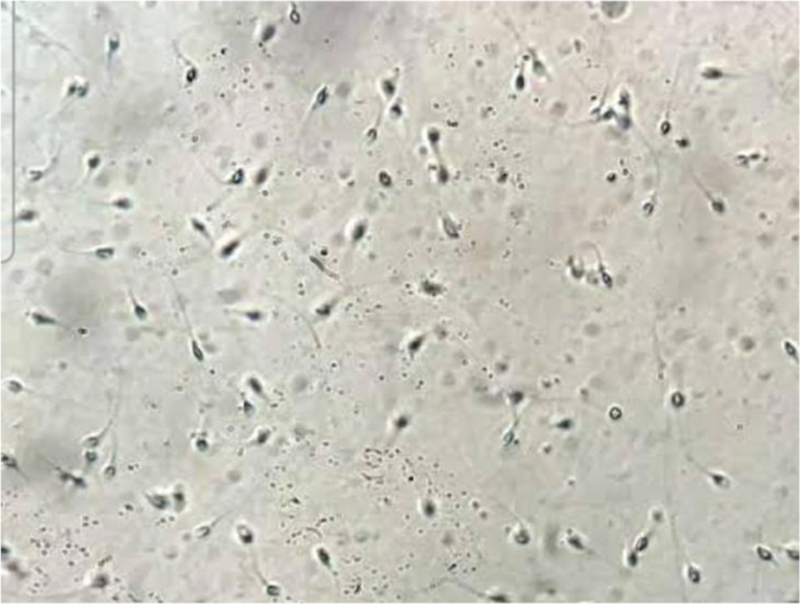
- Sperms seen under magnification after teasing out the seminiferous tubules.
![The transferred blastocysts: 1*412,1*312 [as per European Society of Human Reproduction and Embryology (ESHRE) 2011 Istanbul Consensus].](/content/172/2023/10/4/img/FSAR-10-219-g003.png)
- The transferred blastocysts: 1*412,1*312 [as per European Society of Human Reproduction and Embryology (ESHRE) 2011 Istanbul Consensus].
The second FET preparation has started. On day 18 of endometrial preparation, the endometrial thickness was 9.3mm with blood flow in zone 2 and progesterone level was 0.3. Of the three day three embryos were left to culture till day five of which one embryo got arrested. On the day of transfer two blasts with grades 412 and 312 (as per Istanbul Consensus grading) were transferred.
Outcome
The urine pregnancy test result was positive on May 29, 2023, and the beta Human Chorionic Gonadotropin (HCG) level was 4551 mIU/mL.
The first ultrasound done on June 5, 2023 showed twin intrauterine (dichorionic diamniotic) pregnancy of 5 weeks 5 days.
However, around June 13, 2023, the patient had a bout of bleeding per vagina, for which conservative treatment was given, and a repeat ultrasound reassured that the twin pregnancy was fine but with a subchorionic bleed, which settled with conservative medical management.
Currently, the patient has successfully completed 4 months of twin pregnancy journey with all normal genetic screening parameters.
DISCUSSION
Cases of male infertility must be evaluated systematically, and if surgical sperm retrieval is done by microsurgical method by experts, it has a good outcome. Every case of azoospermia should be counseled about surgical sperm retrieval methods to enable a couple to have their own genetic offspring. Additionally, the option of a repeat micro-TESE after a previous failed surgical sperm retrieval such as TESA or C-TESE by an experienced surgeon should be attempted if the patient can afford it and may be done after administering adjuvant hormonal treatment.
The procedure of micro-TESE was first described by Schlegel, and till date reports have shown sperm recovery rates of up to 45% in cases of NOA with micro-TESE for patients with a history of previously failed C-TESE.[12,13,14]
Hormonal optimization before TESE is still under investigation, and reports are conflicting. A study by Reifsnyder et al. also reported a partial effect of testosterone optimization on micro-TESE outcome following treatment with clomiphene citrate, HCG, or aromatase inhibitors[17] showed no statistical significance in results of treated and untreated men. Another study by Shiraishi et al. where both the first and repeat surgeries after adjuvant hormonal treatment were done by the same surgeon showed success rate between 10 and 21%.[15,16]
Sperm extraction by way of micro-TESE is a technically demanding procedure and is influenced strongly by the experience of the operating surgeon. Surgical outcomes and sperm retrieval were shown to improve as the surgeon gained more surgical experience.[18]
CONCLUSION
This case of azoospermia though clinically and based on hormonal profile appeared to be one of OA. However, a previous vasoepididymal anastomosis surgery did not result in any restoration of sperms in the semen.
Thereafter, a TESA procedure done yielded poor result and the reason could possibly be that, azoospermia was actually NOA with scattered islands of spermatogenesis. Possibly, the surgeon available then was not as experienced and hence retrieval was not satisfactory.
And a subsequent repeat surgical sperm retrieval after administration of HUCOG and by way of micro-TESE procedure and by an experienced surgeon yielded good sperm retrieval, good embryo formation, and a subsequent successful pregnancy.
Hence, conclusions drawn are that
Not all cases of azoospermia are clear-cut cases of obstructive or NOA.
If it is a case of NOA, TESA may or may not yield good results.
If a repeat surgical sperm retrieval is planned in the case of NOA, it is better to be ensured that an experienced surgeon should do it and a repeat surgical procedure should be micro-TESE.
Hormonal optimization may also help with improved sperm retrieval subsequently.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab. 2011;25:271-85.
- [CrossRef] [PubMed] [Google Scholar]
- Canary in the coal mine? Male infertility as a marker of overall health. Annu Rev Genet. 2020;54:465-86.
- [CrossRef] [PubMed] [Google Scholar]
- Concepts in diagnosis and therapy for male reproductive impairment. Lancet Diabetes Endocrinol. 2017;5:554-64.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17-8.
- [CrossRef] [PubMed] [Google Scholar]
- Conventional in-vitro fertilization versus intracytoplasmic sperm injection for patients requiring microsurgical sperm aspiration. Hum Reprod. 1994;9:1705-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancies after testicular sperm extraction and intracytoplasmic sperm injection in non-obstructive azoospermia. Hum Reprod. 1995;10:1457-60.
- [CrossRef] [PubMed] [Google Scholar]
- Causes of azoospermia and their management. Reprod Fertil Dev. 2004;16:561-72.
- [CrossRef] [PubMed] [Google Scholar]
- Report on evaluation of the azoospermic male. Fertil Steril. 2006;86(5 suppl 1):S210-5.
- [CrossRef] [Google Scholar]
- Salvage microdissection testicular sperm extraction after failed conventional testicular sperm extraction in patients with nonobstructive azoospermia. J Urol. 2006;175:1446-9.
- [CrossRef] [PubMed] [Google Scholar]
- Salvage micro-dissection testicular sperm extraction; outcome in men with non-obstructive azoospermia with previous failed sperm retrievals. BJU Int. 2015;116:460-5.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of microdissection testicular sperm extraction, conventional testicular sperm extraction, and testicular sperm aspiration for nonobstructive azoospermia: a systematic review and meta-analysis. Fertil Steril. 2015;104:1099-110.
- [CrossRef] [PubMed] [Google Scholar]
- Human chorionic gonadotrophin treatment prior to microdissection testicular sperm extraction in non-obstructive azoospermia. Hum Reprod. 2012;27:331-9.
- [CrossRef] [PubMed] [Google Scholar]
- Salvage hormonal therapy after failed microdissection testicular sperm extraction: a multi-institutional prospective study. Int J Urol. 2016;23:496-500.
- [CrossRef] [PubMed] [Google Scholar]
- Role of optimizing testosterone before microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol. 2012;188:532-6.
- [CrossRef] [PubMed] [Google Scholar]
- Learning curves of microdissection testicular sperm extraction for nonobstructive azoospermia. Fertil Steril. 2010;94:1008-11.
- [CrossRef] [PubMed] [Google Scholar]







