Translate this page into:
Association between lifestyle factors and semen parameters: An overview of systematic reviews
Address for correspondence: Abha Maheshwari, Aberdeen Fertility Centre, Aberdeen Maternity Hospital, Aberdeen, Scotland, Pin Code AB25 2ZL, UK. E-mail: abha.maheshwari@abdn.ac.uk
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Infertility, defined as inability of a couple to conceive after a year of unprotected regular intercourse, with third of cases due to suboptimal sperm quality. There are modifiable and nonmodifiable risk factors that can affect the quality and quantity of sperm and hence fertility. Several separate systematic reviews exist on this topic and clinicians are often faced with a plethora of reviews with variable quality giving conflicting advice. Therefore, we summarized the current available data by conducting a systematic review of systematic reviews on risk factors such as coffee/caffeine, body mass index (BMI)/obesity, cigarette smoking, and paternal age, on sperm parameters of count, motility, and morphology so that all evidences are present together, at one place. Embase, OVID MEDLINE(R), and Cochrane central database of systematic review were searched for relevant publications between 2010 and present. Search terms were: smoking, obesity, obese, BMI, caffeine, paternal age, advanced paternal age, male infertility, male fertility, sperm motility, sperm quality, and sperm analysis. Systematic reviews that met the criteria were retrieved and the relative reference lists were searched. All included studies were quality assessed using the AMSTAR checklist tool. Electronic and manual hand search yielded a total of 318 studies, of which 11 were excluded after removing duplicates and a further 286 excluded based on titles and abstract. Full-text screening of 21 articles, excluded 10 further studies. Eleven publications were finally included. Obesity and smoking were associated with decline in sperm count and morphology, age with decline in motility and morphology. Caffeine consumption was not associated with changes in any of the three parameters. Obesity and smoking are modifiable risk factors impacting on the semen parameters; caffeine consumption may not have any adverse effects on sperm parameters. This overview was limited by the quality of included reviews which in turn were limited by observational nature of the included studies, small numbers, and heterogeneity of the population. Further prospective data collection is needed to have good quality evidence. In conclusion, high BMI, smoking, and advanced paternal age were found to be associated with decline in one or more parameters of semen quality in males, albeit the evidence is of varying strength. Caffeine was not associated with any deterioration.
Keywords
BMI
caffeine
male fertility
paternal age
semen parameters
smoking
INTRODUCTION
Infertility is defined as inability of a couple to conceive after 12 months of regular unprotected intercourse. One in seven couples is affected across the world. Contributing factors to infertility could be male, female, or combined factors. Approximately 30% of the causes are attributed to the male partner.[1] Semen analysis is a basic and first investigation used to evaluate male fertility. Parameters of semen analysis used to assess sperm are count, motility, and morphology,[2,3,4] as per strict World Health Organization (WHO) criteria.[5,6]
There are various lifestyle factors that can affect the quality and quantity of sperm.[7,8] The modifiable risk factors include obesity/body mass index (BMI), smoking, and caffeine intake, whereas nonmodifiable includes paternal age. Several studies have studied the impact of these modifiable risk factors on sperm count, motility, and morphology.[9]
Overweight and obesity are associated with excess fat accumulation, which can be measured using the BMI, where 25 to 29.9 is overweight and >30 is obese. There are several risks and complications to obesity and reduced fertility is now recognized as one of them.[10] Some studies have found an association between high BMI and low semen volume with no other semen parameters affected.[10,11,12,13]
Smoking cigarettes has been associated with a deterioration of sperm quality. Although the etiology is not fully understood, it is suggested that toxins from cigarette smoke can decrease sperm mitochondrial activity and damage the chromatin structure in human sperm.[9] However, the evidence is controversial, and some studies have found no effect on semen quality.[14,15,16]
Coffee consumption, on the other hand, has been hypothesized to influence not only semen parameters, but also sperm DNA integrity. However, most studies have failed to find an association between amount of caffeine consumption and male fertility.[9]
Finally, as more couples are choosing to delay pregnancy to later stages of life,[17] it is important to understand the impact of advanced paternal age on fertility outcomes. Although increasing maternal age has been established as a factor for fertility,[18] the influence of paternal age is poorly understood. Nevertheless, Johnson et al. report that several studies suggest advanced paternal age is associated with declines in fertility.[19]
Various systematic reviews exist on these topics, some with conflicting conclusions. Hence, clinicians are increasingly faced with difficulties in decision making. Systematic reviews (or overviews) of reviews are a logical and appropriate next step, allowing the findings of separate reviews to be compared, providing clinical decision makers with the evidence they need. Therefore, we summarized the current available data and conducted an overview of systematic reviews on the association between risk factors such as coffee/caffeine, BMI/obesity, cigarette smoking, and advanced paternal age, on semen count, motility, and morphology so that all evidences are present together at one place.
METHODS
The electronic databases Embase, OVID MEDLINE(R), and Cochrane central database of systematic review were searched to identify relevant systematic reviews published from 2010 to October 2020, so only articles post-WHO criteria update were included. Studies prior to this update would have reported semen characteristics of patients as abnormal, whom will now be reclassified as normal based on the new reference values.[6] Search terms used were caffeine OR smoking OR obesity OR BMI OR paternal age AND male (in)fertility OR sperm OR sperm/semen motility OR sperm/semen count/sperm concentration OR sperm/semen morphology. Search was limited to only studies in humans, males, and systematic reviews but no language restriction applied.
Titles and abstracts of all articles retrieved using above-mentioned search terms from the database search were screened. The inclusion criterion was systematic reviews assessing the impact of lifestyle factors such as “smoking,” “obesity,” “BMI,” “advanced paternal age,” “caffeine”/“coffee” on semen parameters of “sperm count,” “sperm motility,” and “sperm morphology.” The studies were excluded if they were not performed on humans and if they mentioned about interventions/treatments/in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI)/supplements.
Full texts of selected abstracts matching inclusion criteria were obtained. In addition, reference lists of included articles were hand searched. Studies were analyzed for inclusion independently by two of the authors (BA and SF). Any discrepancies were resolved by discussion with AM or SV. Articles were included only if full texts were available. The author(s), publication year, aim of study, search strategy, number of studies included, study characteristics, and result outcomes were carefully extracted. The quality of each of the included studies was assessed according to the criteria set by A MeaSurement Tool to Assess Systematic Review (AMSTAR).
RESULTS
The search strategy identified a total of 318 articles, including 121 from Cochrane database of systematic reviews, 113 from Embase, 83 from OVID MEDLINE(R), and 1 article from manual searches of references. However, 11 of these studies were duplicates. After reviewing studies based on titles and abstracts, as they had no relevance to the primary research question, 286 studies were excluded. Twenty-one full text articles were assessed for eligibility. Among the full text articles, further studies were eliminated because of no access to full text (n = 1); only conference abstract (n = 8); outcomes on DNA fragmentation (n = 1) only.
Finally, 11 studies which met all the inclusion and exclusion criteria were included in this present study [Figure 1]. Five were on of BMI, two for smoking, one for advanced paternal age, another one for coffee/caffeine intake, and two with multiple exposure/risk factor groups.

- The flow diagram depicts the flow of information through the different phases of PRISMA literature search. It maps out the number of records identified, included, and excluded and the reasons for exclusion.
A total number of 438 likely overlapping observational studies were included in this analysis. The scope of the original reviews is summarized in Table 1. The study aims, search strategy, characteristics, quality assessment, and study outcomes are listed in Table 1.
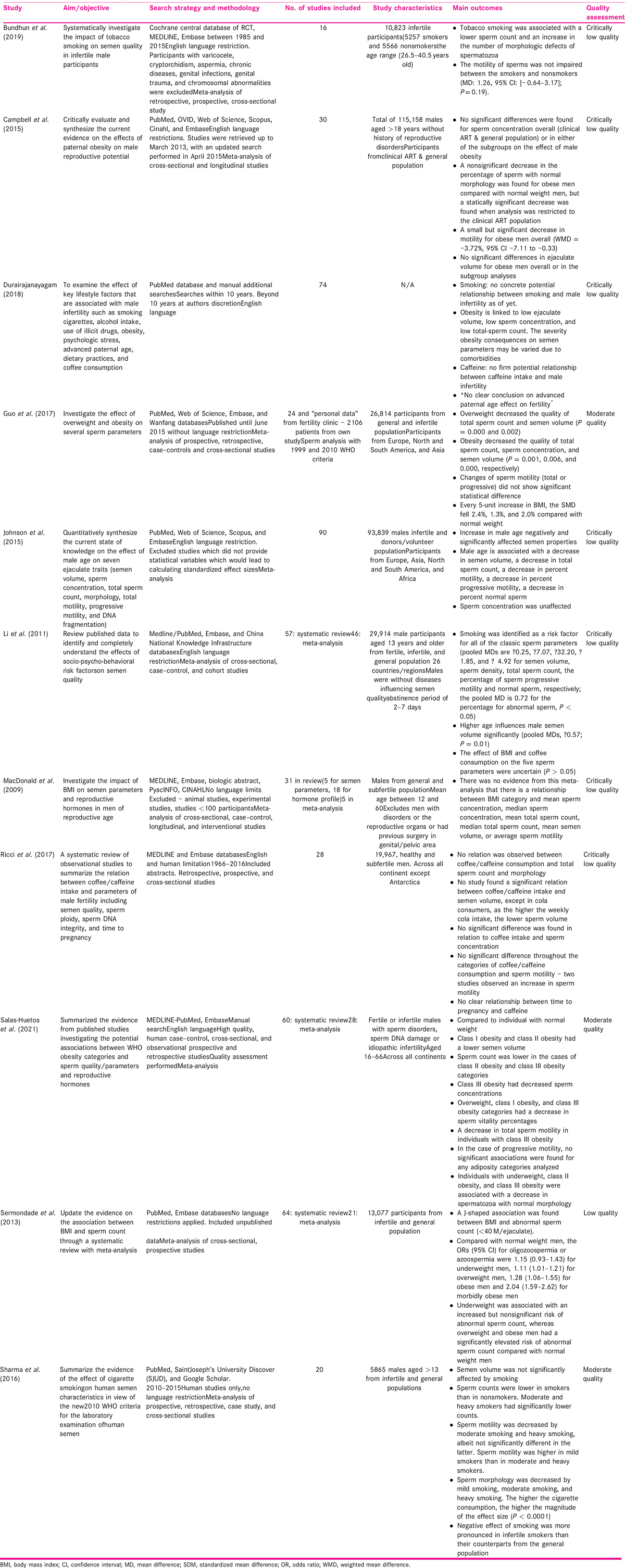
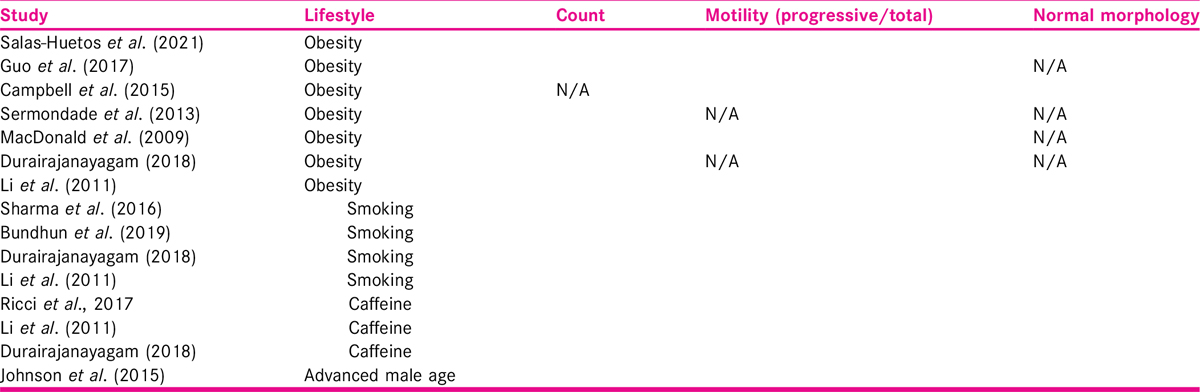
The outcomes of the included studies are presented in Table 2.
Smoking
Four[9,20,21,22] of the included 11 studies investigate smoking as a risk for infertility. The studies ranged in quality, one at moderate quality,[20] whereas others[9,21,22] had critically low quality.
The largest study[21] had 29,914 participants from fertile, infertile, and general population. Participants were aged 13 or above without diseases. Bundhun et al.[22] included 16 individual studies with a total number of 10,823 infertile male participants (5257 smokers and 5566 nonsmokers) and age between 26 and 40 years. Sharma et al.[20] had 20 individual studies (n = 5865). Durairajanayagam[9] had no information on study characteristics but reports the inclusion of 74 studies.
Three studies[20,21,22] out of four reported that smoking was associated with decline in semen parameters. The fourth one[9] concluded that tobacco smoking was associated with a lower sperm count and an increase in the number of morphologic defects of spermatozoa. However, motility of sperms was not impaired between the smoker and nonsmoker groups. Sharma et al.[20] established exposure to cigarette smoking was associated with lower sperm counts and motility in moderate and heavy smokers compared to nonsmokers.
Proportion of normal morphology was decreased even in those who were classified as mild (1–10 cigarettes), moderate (10–20 cigarettes), or heavy (>20 cigarettes) smoking. The higher the cigarette consumption, the higher the magnitude of the effect size (P < 0.0001). Overall negative effect of smoking was more pronounced in infertile smokers than their counterparts from the general population. Smoking was associated with reduced total sperm count, progressive motility, and normal morphology in study by Li et al.[21]as well.
Furthermore, Sharma et al.[20] found the overall effect on sperm count and motility remained similar when the 2010 and earlier WHO manuals were used, but it differed with regard to sperm morphology. Although the review set out to investigate the effect of the new criteria, some of the studies included were published prior to 2010 and the discrepancy was correlated to the length of time and compliance it takes to adopt guidelines into routine practice.
Conversely, Durairajanayagam[9] reported the lack of concrete significant evidence to support the potential relationship between smoking and male infertility. However, available evidence from previous studies support the recommendation of smoking cessation and minimizing exposure to tobacco smoke among couples who are trying to conceive.
Overall, a majority of these studies suggest a smoking history has a significant negative impact on semen parameters. Reduction in sperm count, motility, and normal morphology are associated with smoking, where the degree of abnormality is based on the smoking pack years.
Almost all individual studies included in these reviews were small; they were based on retrospective data collection of routinely collected data, hence limited by design.
Neither of them looked at newer methods of smoking such as vaping or e-cigarettes or use of nicotine patches.
Body mass index
Seven of the included 11 studies investigate BMI as a risk factor for infertility of which 2 were moderate quality, 1 was low, and 4 were critically low quality. Dates of publication ranged from 2011 to 2020.
Participants included general population and those attending infertility clinics[21,23,24,25,26]; from only infertility clinic[27] or were not clear.[9] Number of included studies varied from 24 to 74 and population size varied from <100 to 115,158 but size was not reported in three studies.[9,26,27] Campbell et al.[24] had the largest population size (n = 115,158) where participants were aged 18 or above without history of reproductive disorders. In addition, a tenth of the data reported in Guo et al.[25] and 5% in Sermondade et al.[23] were data from unpublished studies.
Three studies[23,25,26] used WHO criteria for obesity and further divided into three subgroups of 30.0 to 34.9 kg/m2 (class I obesity), 35 to 39.9 kg/m2 (class II obesity), and ≥40.0 kg/m2 (morbid obesity, or class III obesity).
Campbell et al.[24] divided participants into two groups: normal BMI and obese, but missed the overweight group. Macdonald et al.[27] had three WHO categories of normal, overweight, and obese. BMI categories were uncertain in others.[9,21] In addition, Durairajanayagam[9] was a descriptive review with limited information about BMI categories.
Association of sperm count with high BMI was reported by all studies except one[24]; with four[9,23,25,26] suggesting reduction; and two[21,27] reporting no difference. Association of sperm motility with high BMI was reported by five studies, with two studies[24,26] suggesting reduction; and three[21,25,27] reporting no difference. Reduction of normal morphology with high BMI was reported by two studies[24,25] and one[21] reporting no difference. None of the seven studies reported a relationship between a higher BMI and a worse decline in any of the semen parameters. As is visible from Table 2, there is consistent pattern of reduction in count and normal morphology but not in motility.
Coffee/caffeine
Three[9,21,28] of the 11 included studies investigated the effect of caffeine on male fertility, and all three were of critically low quality published in 2011, 2017, and 2018.
Ricci et al.[28] included fertile and subfertile males. This review included 28 individual studies (n = 19,967). The individual studies varied in size from 41 to 4474 participants. Source of caffeine varied, so as the population studied. This review concluded that semen parameters did not seem to be affected by caffeine intake, at least caffeine from coffee, tea, and cocoa drink.
Advanced paternal age
Three of the included 11 studies[9,19,21] explore the relation between advanced paternal age/aging on semen parameters. Of which, a study by Johnson et al.[19] is the most comprehensive with 93,839 males, from 90 individual studies, population consisting of infertile and donors as well as volunteer population. Of the three, this is the only one[19] with primary focus on age and semen quality. All three studies were of critically low quality.
All studies included in Li et al.[21] were already included in Johnson et al..[19] Li et al.[21] only assessed semen volume and not count, motility or morphology. Review by Durairajanayagam[9] was the latest publication of all the three and had systematic searches but had limited details about impact of age on semen parameters. It has description of few individual studies.
The meta-analysis in Johnson et al.[19] suggests that increase in male age was found to be associated with a decrease in total and progressive motility, and percent normal sperm morphology but there was no impact on sperm concentration. All individual studies included in this review[19] were small (range n = 25–3669). This is based on published retrospective data, hence unable to adjust for confounders or use age as a continuous variable. Hence, it was not possible to define a specific age where decline in motility happens or rate at which decline happens.
DISCUSSION
Main findings
This study reviewed all the available systematic reviews in last 10 years, which investigated the effect of four lifestyle risk factors on three sperm parameters using a systematic review approach. Three of these factors including BMI, smoking, and advanced paternal age were identified as significant risk factors for semen quality, but the effect of coffee/caffeine consumption on semen quality was not significant. BMI was the most studied lifestyle factor. Smoking has an adverse effect on semen parameters and fertility, where sperm count, motility, and normal morphology are significantly reduced. In addition, the amount of cigarette smoked is suggested to be related to the degree of semen parameter decline and male infertility. Advancing paternal age results in decline of sperm parameters such as sperm count, total and progressive motility, and normal morphology. Increased BMI was associated with decreased total sperm count compared to normal weight men. A small significant decrease was found in sperm motility for obese men overall but no statistical difference for progressive motility. A statistical decrease was reported in the percentage of normal sperm morphology in obese subgroups, especially when restricted to the population undergoing assisted reproduction.
Strength
The main strength of this systematic review is its comprehensive literature search. It is also the first overview of systematic review to summarize all the available data. We also only included review studies published after the latest WHO criteria to reflect the best and most up to date result.[6]
Limitations
Our study has few limitations. First, the extreme heterogeneity on exposure measurements, study populations, and outcomes make it difficult to draw concrete conclusions on the effects of lifestyle factors on fertility. Second, this systematic review of reviews found very few studies summarizing the relationship between risk factors and semen parameters, of which a majority were of poor quality. Over 70% of the included studies were of low or critically low quality when assessed. In addition, a significant number of the reviews failed to assess the risk of bias of the individual studies. Hence, the results should be interpreted with caution. Thirdly, the measurement of BMI and smoking was not ideal. These were self-reported that could be under- or overestimated, and the included reviews failed to quantify smoking. There were no reviews reporting on e-cigarettes or vaping. We have not looked at sperm DNA fragmentation. Although DNA fragmentation may show evidence of sperm damage, there is no evidence for its use as a routine diagnostic test in clinical practice. We have limited ourselves to three most important parameters of semen analysis as advocated by WHO. We appreciate that DNA fragmentation has been used widely especially in nonpublic sector; however, it is unclear whether it is a diagnostic or a prognostic test, there is a lack of gold standard, there is no consensus on its interpretation with multiple assays, let alone no treatment if sperm DNA fragmentation is identified. Hence, it was not appropriate to study the impact of lifestyle factors on sperm DNA fragmentation within this review.
The BMI was used in the systematic reviews as it is the conventional measure of obesity. However, it is an imperfect and indirect measure of body fat, especially when the thresholds have been questioned[29] and measure is based on self-reported height and weight[30] as is the case in the included review of this present study.
This review concentrated on effect of lifestyle factor on semen parameters. Semen parameters do not automatically mean reduced male fertility as semen analysis as it is, has a very poor positive predictive value as a test of fertility.[31]
CONCLUSION
In conclusion, high BMI, smoking, and advanced paternal age were found to be associated with decline in one or more parameters of semen quality in males, albeit the evidence is of varying strength. Caffeine was not associated with any deterioration.
Implications for policy and practice
Although there is association with decline in sperm count and motility with obesity, there are perceptions that as long as there are some motile sperms, assisted reproductive treatments such as IVF with addition of ICSI can be performed. Hence male age is not included in the access criteria. However, in addition to being expensive and invasive, these treatments are associated with complications such as ovarian hyperstimulation for women, increased risk of obstetric and perinatal complications when compared with those of spontaneous conception. Public funding of these treatments is limited as well across the world with most needing to pay themselves. Consequently, they should only be used when necessary and not to compensate for the lifestyle factors.
It is therefore really important that investment is carried out in education about the risk of these modifiable factors and their association with reduction in semen parameters. These could be one of few preventable causes of male infertility. Every opportunity for such education should be used.
Implications for future research
Seven out of 11 reviews are of critically low quality: others low or moderate quality. This is because of observational nature and routinely collected data. Randomized data will never be available to answer this question. To get high-quality observational data, well-designed studies with predefined criteria for semen analysis and for subject selection as well as clear definition of lifestyle factor are essential to reach a strong conclusion.
Prospective validated data need to be collected for all men undergoing semen analysis. There has to be international consensus for such data collection. It is only then we can assess the strength of association in large number and in different population. We also must be mindful that these data can only assess the association and not causation.
Authors’ contributions
AM conceived the study, BA and SF did the searches, extracted data and quality assessments. BA wrote the first draft of the manuscript. All authors contributed to initial and final draft.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Trends in the use of intracytoplasmatic sperm injection marked variability between countries. Hum Reprod Update. 2008;14:593-604.
- [Google Scholar]
- Semen quality of male smokers and nonsmokers in infertile couples. Fertil Steril. 2003;79:287-91.
- [Google Scholar]
- Semen quality of smoking and non-smoking men in infertile couples in a Turkish population. Arch Gynecol Obstet. 2005;271:109-12.
- [Google Scholar]
- Smoking practices in Jordanian people and their impact on semen quality and hormonal levels among adult men. Cent Eur J Public Health. 2011;19:54-9.
- [Google Scholar]
- Examination and Processing of Human Semen. World Health; 2010. p. :286. Edition V(10). Available at http://whqlibdoc.who.int/publications/2010/9789241547789_eng.pdf
- The effect of the new 2010 World Health Organization criteria for semen analyses on male infertility. Fertil Steril. 2012;98:1428-31.
- [Google Scholar]
- Modifiable and non-modifiable risk factors for poor sperm morphology. Hum Reprod. 2014;29:1629-36.
- [Google Scholar]
- Modifiable and non-modifiable risk factors for poor semen quality: a case-referent study. Hum Reprod. 2012;27:2799-806.
- [Google Scholar]
- The clinical biochemistry of obesity. The Clinical biochemist. Reviews. 2004;25:165-81. Available at http://www.ncbi.nlm.nih.gov/pubmed/18458706%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1880830
- [Google Scholar]
- Healthy overweight male partners of subfertile couples should not worry about their semen quality. Fertil Steril. 2010;94:1356-9.
- [Google Scholar]
- An exploration of the association between male body mass index and semen quality. Reprod BioMed Online. 2011;23:717-23.
- [Google Scholar]
- High body mass index has a deleterious effect on semen parameters except morphology: results from a large cohort study. Fertil Steril. 2014;102:1268-73.
- [Google Scholar]
- The impact of cigarette smoking on human semen parameters and hormones. Hum Reprod. 2002;17:1554-9.
- [Google Scholar]
- Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect. 2003;111:414-20.
- [Google Scholar]
- Semen quality of1346 healthy men, results from the Chongqing area of southwest China. Hum Reprod. 2009;24:459-69.
- [Google Scholar]
- Effect of female age on the diagnostic categories of infertility. Hum Reprod. 2008;23:538-42.
- [Google Scholar]
- Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22-33.
- [Google Scholar]
- Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur Urol. 2016;70:635-45.
- [Google Scholar]
- Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril. 2011;95:116-23.
- [Google Scholar]
- Tobacco smoking and semen quality in infertile males: a systematic review and meta-analysis. J Urol. 2019;202:446.
- [Google Scholar]
- BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19:221-31.
- [Google Scholar]
- Paternal obesity negatively affects male fertility and assisted reproduction outcomes: a systematic review and meta-analysis. Reprod BioMed Online. 2015;31:593-604.
- [Google Scholar]
- The impact of BMI on sperm parameters and the metabolite changes of seminal plasma concomitantly. Oncotarget. 2017;8:48619-34.
- [Google Scholar]
- Male adiposity, sperm parameters and reproductive hormones: an updated systematic review and collaborative meta-analysis. Obes Rev. 2021;22:1-33.
- [Google Scholar]
- The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2009;16:293-311.
- [Google Scholar]
- Coffee and caffeine intake and male infertility: a systematic review. Nutr J. 2017;16:1-14.
- [Google Scholar]
- Do reproductive hormones explain the association between body mass index and semen quality? Asian J Androl. 2007;9:827-34.
- [Google Scholar]
- Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102:1502-7.
- [Google Scholar]







