Translate this page into:
Cytokines as a Stress Marker in Infertility Patients – A Perspective

*Corresponding author: Goli Shiva Krishna, Department of Reproductive Medicine, Virinchi People’s Hospitals, Hyderabad, India. shivaemb18@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Krishna GS. Cytokines as a Stress Marker in Infertility Patients – A Perspective. Fertil Sci Res. 2025;12:5. doi: 10.25259/FSR_39_2024
Abstract
Psychological stress might play a crucial role in infertility and miscarriage. Infertile women have greater vulnerability to psychological stressors, which may lead to major depressive disorder. Psychological stress acts as a trigger for anxiety and depression disorders. Acute or chronic stress could act as a risk factor and lead to increased production of pro-inflammatory cytokines, which causes changes in the neurotransmitter system and may be involved in the development of depression. Some studies have shown that pro-inflammatory cytokines can be elevated during psychological stress. However, their reliability as a stress marker is still in query.
Keywords
Cytokines
Infertility
Stress
INTRODUCTION
Psychological stress might play a crucial role in positive outcomes in infertile patients. Infertile women have greater vulnerability to psychological stressors, which may lead to major depressive disorder (MDD).[1,2] Psychological stress acts as a trigger for anxiety and depression disorders.[1,2] Sterile inflammation is a kind of inflammation due to the absence of external pathogens. Psychological stress is an example of sterile inflammation.[3] Acute or chronic stress could act as a risk factor and lead to increased production of pro-inflammatory cytokines, which causes changes in the neurotransmitter system and may be involved in the development of depression.[4]
Research on Wistar rats, when subjected to stress, was reported with elevated levels of tumour necrosis factor (TNF -alpha) and interlukin-10 (IL-10), IL-6, IL-4, and IL-2 levels when compared with the restraint rats.[5] Hamovici et al. have evaluated 53 infertile couples. Stress, anxiety, and depression have been evaluated through assessment scales. Cytokines have been checked from blood serum, semen, cervicovaginal lavage, and follicular fluid on the day of oocyte retrieval. Low serum TGF-B and high levels of IL-1B and IL-6 were reported in couples who are undergoing stress. A strong correlation has been observed between cytokines in relation to stress and depression in both partners.[6]
Cumulative evidence has suggested an association between psychosocial stress and systemic inflammation, which is estimated by inflammatory cytokines.[7] Increased inflammation has frequently been associated with psychosocial stressors. Clinical and laboratory evidence suggests that the main way stress exposure influences the aetiology of these psychopathologies is through its capacity to trigger and elevate the levels of pro-inflammatory cytokines IL-6, TNF-alpha and IL-1B levels.[8]
MOLECULAR PATHOPHYSIOLOGY OF CYTOKINES IN THE BRAIN DURING STRESS
The most ubiquitous inflammasome, nucleotide-binding oligomerisation domain, leucine-rich repeat, and pyrin domain protein 3 (NLRP3) is triggered by two signals in the nuclear factor kappa-B (NFkB) signalling pathway during psychological stress. One significant signalling mechanism that interferes with the production of inflammatory intercessors TNF-alpha, IL-6, and ProIL-1B is the NFkB signalling system. The NFkB signalling system is initiated by the endogenous ligands high mobility group box-¹ (HMGB¹) and heat shock protein 72 (Hsp72).[9–11] Enzyme procaspase 1, along with caspase recruitment domain (ASC) and apoptosis-associated speck-like protein, forms a complex. ASC and pro-caspase 1 complex binds with NLRP3 and forms the second activating signal, which cleaves Pro-IL-1b is cleaved into IL-1b. Alternatively, pro-IL-1b (and pro-IL-18) are cleaved into their active forms by other intracellular multiprotein complexes.[12] The most common inflammasome to be activated following psychological stress is the NLRP3 inflammasome. The ATP released by astrocytes shapes NLRP3 inflammasomes. NLRP3 inflammasome is one of the main danger signals at purinergic type 2X7 receptors (P2X7Rs).[11,13] Overproduction of ATP triggers microglia and over-activation of NLRP3 inflammasome and neuroinflammation.[14]
ACTIVATION OF THE HPA AXIS DURING PSYCHOLOGICAL STRESS
Psychological stress provokes an immune response that instigates microglia in the central nervous system to release pro-inflammatory cytokines and triggers the hypothalamic-pituitary axis (HPA) [Red arrows – Figure 1]. Pro-inflammatory cytokines secreted by peripheral immune cells evoke all three levels of the HPA axis, including the microglia in the central nervous system, via activation of the vagus nerve.[15]
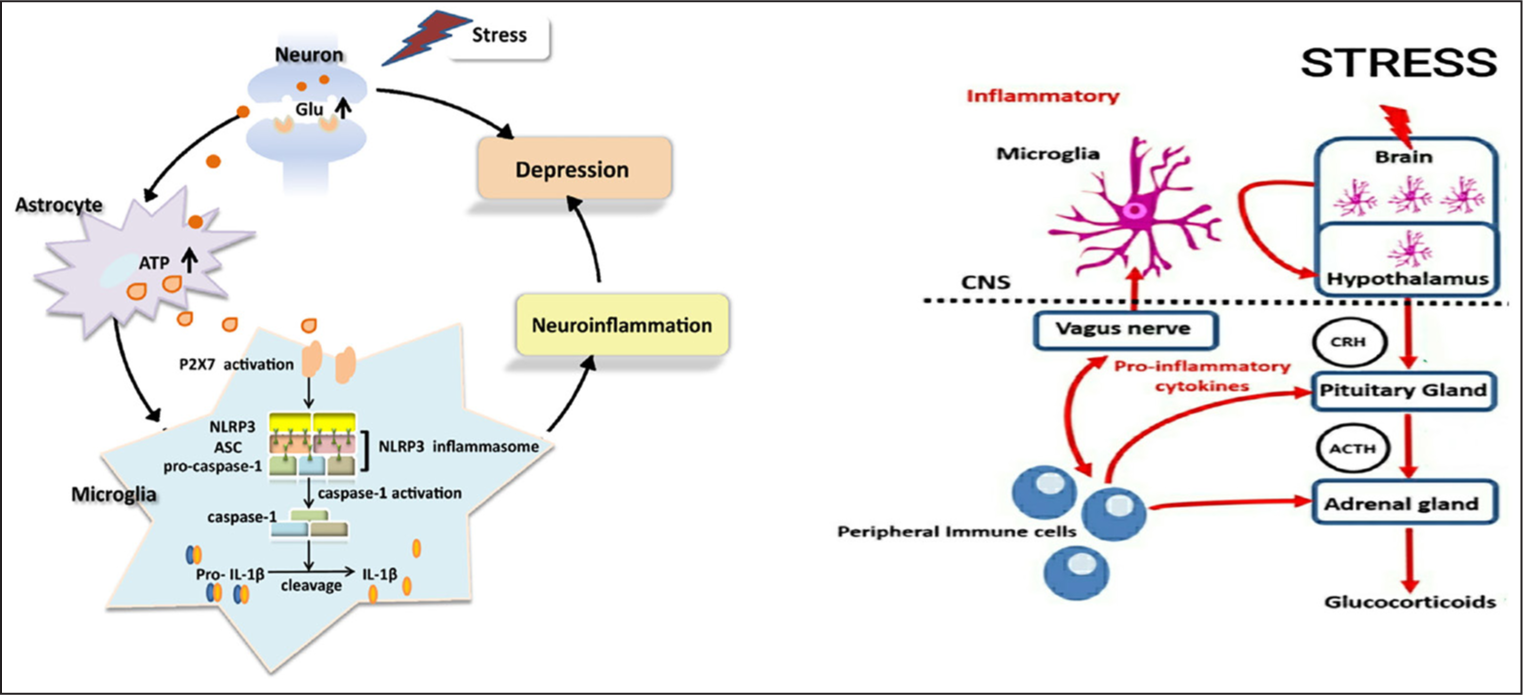
- The P2X7–NLRP3–IL-1β pathway is the most prevalent stress mechanism that causes pro-inflammatory cytokines to be released. Excess Glu is produced and released at the synaptic terminals of neurons in response to stress, which encourages astrocytes to release more ATP. When cytokines are released, the HPA axis is activated. Glu: Glutamate; P2X7: Purinergic P2X receptor 7; Pro-IL-1B: Pro-interleukin-1B; CNS: Central nervous system; CRH: Corticotropin-releasing hormone; ACTH: Adrenocorticotropic hormone; ATP: Adenosine triphosphate; NLRP3: Nucleotide-binding oligomerization domain, Leucine-rich repeat, and pyrin domain protein 3; ASC: Apoptosis-associated speck-like protein containing a caspase recruitment domain; IL-Iβ: Interlukin-1 beta.
CRH SHOWING ITS EFFECT ON HPG AXIS
Psychological stress activates the sympathomedullary system within seconds and releases adrenaline and noradrenaline, which causes an increase in blood supply and heart rate to accommodate attention and combat stress.[13] Activation of immune responses via the HPA axis takes place within minutes after sympathomedullary activation.[15]
The hypothalamic GnRH pulse generator is one of the most important structures that gets inhibited during stress, and the severity depends on the kind of stress involved (acute or chronic). Stress triggers the release of corticotropin-releasing hormone (CRH) and further activates adrenocorticotropic hormone (ACTH).[14] ACTH triggers the release of cortisol, which regulates stress. A rise in CRH acts by a negative feedback mechanism on the GnRH pulse generator, leading to suppression of the hypothalamic-gonadal axis (HPG). The effect on the HPG axis leads to decreased follicle-stimulating hormone (FSH) and luteinising hormone (LH), which results in steroidogenesis and diminished oocyte maturation in women. Locus coeruleus is activated during stress by the paraventricular nucleus, part of the hypothalamus, that releases noradrenaline, which further triggers the HPA axis [Figure 2].[16]
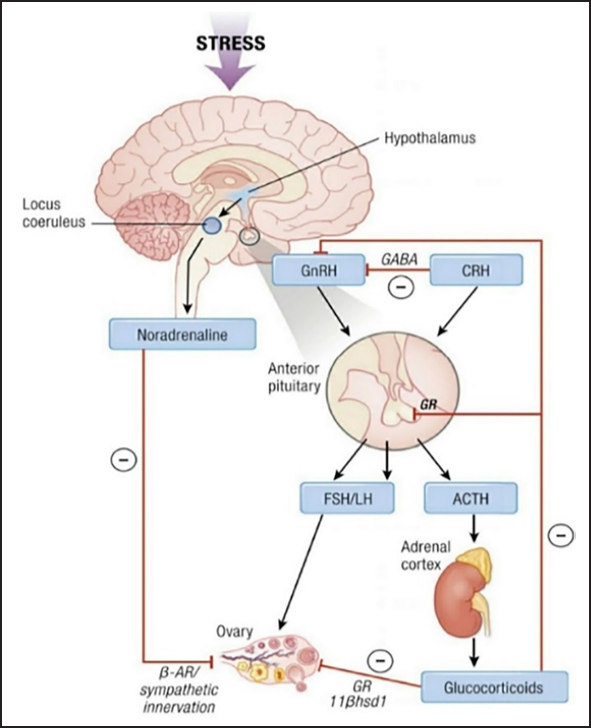
- HPA axis affected by stress, which in turn results in the release of low levels of GnRH. A low level of GnRH influences FSH and LH on the HPG axis. HPA axis: Hypothalamo pituatary adrenal axis; HPG axis: Hypothalamo pituatary gonadal axis; FSH: Follicle stimulating hormone; LH: Luteinizing hormone; B-AR: Beta adrenergic receptor; GnRH: Gonadotropin-releasing hormone; GABA: Gamma amino butyric acid; CRH: Corticotropin-releasing hormone; LH: Luteinising hormone; ACTH: Adrenocorticotropic hormone.
DISCUSSION
Psychological stress induces pro-inflammatory cytokines, which may lead to depression. Nevertheless, behavioral scientists have scrutinized inflammatory markers as an evaluation of the pathophysiology of psychosocial disease. There is concern regarding the standardisation of inflammatory markers, as there are many factors that can alter inflammatory markers. Inflammatory biology is altered with age, and a considerable amount of research suggests that pro-inflammatory cytokines like interleukin-1 receptor antagonist (IL-1ra), IL-6, and TNF-alpha levels increase with age in both women and men. Hormonal factors may impact women’s immune system, which includes the stage of the menstrual cycle, menopausal status, use of hormone replacement therapy (HRT), and use of oral contraceptives (OC).[15] The effect of HRT treatment is more fluttering in women for TNF-α, IL-6, and sIL-6r. Pro-inflammatory cytokines may vary with ethnicity, where IL-6 checked in African Americans showed higher levels than in White Americans. Ho et al.[16] reported that Mexican Americans have increased levels of TNF-α and decreased sTNFR than white Americans. Studies reported that obesity and waist and hip ratio regulated plasma levels of cytokines irrespective of age. An increase in obesity has shown an increase in circulating inflammatory markers TNF-α and IL-6. Acute exercise has shown an impact on inflammatory cytokines.[15] Smoking tobacco has been associated with a rise in IL-6, and in contrast to that, there is a fall in TNF-α levels. Alcohol and a rise in pro-inflammatory cytokines show a U- or J-shaped pattern as the results depend on the amount of alcohol intake. Sleep and circadian rhythms have shown much impact on pro-inflammatory cytokines. IL-6 levels are at higher levels during the day and lower levels during the night. Sleep deprivation leads to a decrease in IL-6 and an increase in IL-1B levels. Medications like aspirin, SSRIs, antihypertensives, and statins may alter the levels of inflammatory cytokines. Altered levels of inflammatory cytokines have been reported with diet and caffeine.[17,18]
CONCLUSION
Psychological stress might show an impact on infertile couples, but many factors control inflammatory cytokines. Inflammatory cytokine levels change with age, sex, BMI (body mass index), hormonal status, diet, exercise, smoking, tobacco, and medications like aspirin, SSRIs, anti-hypertensives, and statins. Currently, the evaluation of inflammatory cytokines should not be considered as a stress marker, as many factors control it and need further clarity for standardisation and co-morbid conditions like cardiovascular diseases. Diabetes also shows a rise in inflammatory cytokines, which also needs to be considered and needs further studies. Many factors could alter cytokines and their types, which need further investigation.
Author contributions
GSK: Carried out all the work including the piling of the whole article and writing of the manuscript.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patients consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Anxiety, Depression and Anger Suppression in Infertile Couples: A Controlled Study. Hum Reprod. 2002;17:2986-94.
- [CrossRef] [PubMed] [Google Scholar]
- The Prevalence and Predictability of Depression in Infertile Women. Fertil Steril. 1992;58:1158-63.
- [PubMed] [Google Scholar]
- Proteose Intoxications and Injury of Body Protein: IV. The Metabolism of Dogs with Sterile Abscess, Pancreatitis, and Pleuritis. J Exp Med. 1918;28:223-41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A Review of the Relationship Between Pro-Inflammatory Cytokines and Major Depressive Disorder. J Affect Disord. 2014;169:15-20.
- [CrossRef] [PubMed] [Google Scholar]
- Stress-Induced Cytokine Changes in Rats. Eur Cytokine Netw. 2013;24:97-103.
- [CrossRef] [PubMed] [Google Scholar]
- Stress, Anxiety, and Depression of Both Partners in Infertile Couples are Associated with Cytokine Levels and Adverse IVF Outcome. Am J Reprod Immunol. 2018;79:e12832.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic Stress, Glucocorticoid Receptor Resistance, Inflammation, and Disease Risk. PNAS. 2012;109:5995-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Neuroendocrine Regulation of Brain Cytokines After Psychological Stress. J Endocr Soc. 2019;3:1302-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Exosomes, DAMPs and miRNA: Features of Stress Physiology and Immune Homeostasis. Trends Immunol. 2017;38:768-76.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Stress Sounds the Alarmin: The Role of the Danger Associated Molecular Pattern HMGB1 in Stress-Induced Neuroinflammatory Priming. Brain Behav Immun. 2015;48:1-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Stress Induces the Danger-Associated Molecular Pattern HMGB-1 in the Hippocampus of Male Sprague Dawley Rats: A Priming Stimulus of Microglia and the NLRP3 Inflammasome. J Neurosci. 2015;35:316-24.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Inflammasomes: Mechanism of Assembly, Regulation and Signalling. Nat Rev Immunol. 2016;16:407-20.
- [CrossRef] [PubMed] [Google Scholar]
- The Inflammasome: Pathways Linking Psychological Stress, Depression, and Systemic Illnesses. Brain Behav Immun. 2013;31:105-14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Glucocorticoid Programming of Neuroimmune Function. Gen Comp Endocrinol. 2018;256:80-8.
- [CrossRef] [PubMed] [Google Scholar]
- Neuroendocrine Interactions of the Stress and Reproductive Axes. Front Neuroendocrinol. 2021;63:100928.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Circulating Tumor Necrosis Factor Alpha is Higher in Non-obese, Non-diabetic Mexican Americans Compared to Non-Hispanic White Adults. Cytokine. 2005;30:14-21.
- [CrossRef] [PubMed] [Google Scholar]
- P2X7 Receptor Mediates NLRP3 Inflammasome Activation in Depression and Diabetes. Cell Biosci. 2020;10:28.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- To Assess, To Control, To Exclude: Effects of Biobehavioral Factors on Circulating Inflammatory Markers. Brain Behav Immun. 2009;23:887-97.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







