Translate this page into:
Efficacy of Co-Q10, vitamin D3, selenomethionine, astaxanthin in reduction of sperm DNA
Address for correspondence: Dhiraj Singh Rananwat, Research Scholar, Department of Biotechnology, BN University, Udaipur, Rajasthan, India. E-mail: dheru198@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
High sperm DNA fragmentation can cause decreased fertility and implantation rate or can cause recurrent implantation failure. If fetus is born, it can lead to genetic abnormalities in the fetus such as Down syndrome, Edward syndrome, or Patau syndrome revealed by Schlegel.[1] The mechanisms examined include: apoptosis in the seminiferous tubule epithelium, defects in chromatin remodeling during the process of spermiogenesis, oxygen radical-induced DNA damage during sperm migration from the seminiferous tubules to the epididymis, the activation of sperm caspases and endonucleases, damage induced by chemotherapy and radiotherapy, and the effect of environmental toxicants. The objective of the study is to evaluate the efficacy of the combination of coenzyme-Q10 (Co-Q10) (100 mg), vitamin D3 (2000 I.U.), selenomethionine (400 mcg), astaxanthin (8 mg) tablet in reduction of sperm DNA fragmentation in males who visited the hospital. The result shows that factors affecting men with high levels of DNA fragmentation will have significantly lower odds of conceiving naturally or through procedures such as intrauterine insemination and IVF. Therefore, in men with high levels of DNA fragmentation, reduced with age and were found to have improvement with 6% to 7% in the age group 30 to 40, > 40 respectively. The Institutional Ethics Committee approved the study procedure (Ref No: SRMC/RP/4505).
Context:
Aim The aim of the study is to evaluate the effect of combination of Co-Q10 (100 mg), vitamin D3 (2000 I.U.), selenomethionine (400 mcg), astaxanthin (8 mg) on sperm DNA fragmentation index (DFI).
Settings and Design:
A type of prospective cohort study in which health conditions are measured before and after the treatment.
Methods and Material:
Patients were recruited after obtaining a written informed consent. On first visit, patient information was taken according to patient information sheet. Patients were counseled to take medication containing antioxidant and vitamin (Co-Q10, vitamin D3, selenomethionine and astaxanthin) for 3-month duration, twice-daily according to general information sheet and fill IPCC (Investigational product compliance card). On the next visit after 3 months, patient information is recorded; and semen examination and sperm DFI were done to note any changes.
Statistical analysis used:
To calculate sample sizes for comparing two means, we have taken confidence interval 95%, power 80%, and ratio of sample size for before and after group as 1. Mean and standard deviation were taken from published data for both the groups as 22.1 ± 7.7 before treatment and 9.1 ± 7.2 after treatment.
Results:
The percent wise distribution of infertile patients was 31.84%, 31.92%, 31.46% from the age group 20 to 30, 30 to 40, and > 40. The maximum number of patients were from the age group 30 to 40 and > 40; hence, control patients were selected from the same age group with percentage 25.01% and 25.35%, respectively.
Conclusions:
Efficacy of Co-Q10, vitamin D3, astaxanthin, and selenomethionine combination in male subjects with raised sperm DFI improved the outcome of assisted reproductive technique. There is decrease in DFI measured in percentage after giving CoQ, which was significant.
Keywords
DNA fragmentation
Co-Q10 Tablets
Male Infertility
In Vitro Treatment
Key Messages:
Men with high levels of DNA fragmentation will have significantly lower odds of conceiving naturally or through procedures such as intrauterine insemination and IVF. Multinutrients may be beneficial for such patients. An elevated sperm DNA fragmentation index has been linked to male infertility. Nutritional aspects may enhance the nuclear DNA integrity of sperm and hence increase the likelihood of conception. Assessment of the impact of supplemental micronutrient intake on subfertile men’s sperm DNA integrity and consequent pregnancy rates is illustrated in this paper.
INTRODUCTION
Infertility is a reproductive condition defined as the inability to generate a clinical pregnancy after at least 12 months of unprotected sexual activity.[1] It could be due to female factors (35% to 40% of couples), male factors (20% to 40% of couples), both (20% to 30% of couples), or it could be unexplained.[2] Ovulatory dysfunction, tubal blockages, and/or endometriosis are the most common causes in women.[3] Chromosomal abnormalities include the presence or absence of whole chromosomes (numerical chromosomal abnormalities), as well as the gain or loss of a whole or portion of chromosome (structural chromosomal abnormalities), all of which cause aneuploidy at various genetic level.[4] Aneuploidy is one of the most prevalent chromosomal abnormalities, accounting for a significant amount of human morbidity and mortality, infertility, and pregnancy loss.[5] Errors in maternal and paternal meiotic chromosomal segregation are the cause of deformities linked to male infertility. However, studies of the effects of coenzyme-Q10 (Co-Q10)[6,7] intake on seminal plasma antioxidant levels are limited. Our findings are congruent with those who showed that Co-Q10 administration (200 mg/day for 3 months) increased superoxide dismutase (SOD) and catalase (CAT) activity in the seminal plasma of infertile men, a positive correlation was found between seminal Co-Q10 concentrations and semen parameters. In contrast, in a study by Alahmar,[8] basal Co-Q10 levels in seminal fluid did not appear to be associated with sperm quality parameters or total antioxidant capacity (TAC).[9,10,11] The increased TAC, SOD, and CAT activities in our study could partially explain the improvement in sperm parameters after treatment with Co-Q10. Although Co-Q10 has been recognized as a powerful mediator of cell signaling, no specific mechanisms have been identified concerning Co-Q10 mediated up regulation of TAC, SOD, and CAT activities.[12,13,14] Interestingly, Co-Q10 has been observed to prevent proinflammatory signaling by insulin, interleukin-17, and STAT3, as well as tumour necrosis factor alpha and various chemokines. In turn, this may reflect an enhanced antioxidant status.[15,16,17] Future investigations are needed to elucidate the mechanisms of the Co-Q10 mediated enhancement of antioxidant capacity.
The present study is focused on combination of the peripheral data of the patients to evaluate the safety and efficacy of “Combination of Co-Q10 (100 mg), Vitamin D3 (2000 I.U.), Selenomethionine (400 mcg), Astaxanthin (8 mg)” tablet in reduction of sperm DNA fragmentation index (DFI).
SUBJECTS AND METHODS
Research Question Is there any impact to recombinant tablet (combination of Co-Q10 [100 mg], vitamin D3 [2000 I.U.], selenomethionine [400 mcg], astaxanthin [8 mg]) on DFI and infertility of male?
Design–A pilot study
Duration − January 2019 to April 2020
Type of Study − A type of prospective cohort study in which health conditions are measured before and after the treatment.
Sample Size − To calculate sample sizes for comparing two means, we have taken confidence interval 95%, power 80%, and ratio of sample size for before and after group as 1. Mean and standard deviation were taken from published data for both the groups as 22.1 ± 7.7 before treatment and 9.1 ± 7.2 after treatment.
Sample size for each group using above formula:
Arm 1 (Before treatment): 39
Arm 2 (After treatment): 39
But as we have used a combined formulation containing four components, we have taken four times the sample 48.
Study Site − Indira IVF Hospital, Udaipur.
Population
Participants
Male patients above the age of 21 years, in a monogamous, heterosexual marriage trying to conceive but having difficulty with conception.
Patients with Sperm DNA Fragmentation Index ≥15% (method of measurement and reference for DFI levels) at the time of screening.
Patients with normal or clinically nonnormal seminal parameters not limited to leucospermia.
Patients with primary or secondary infertility.
Patients who are willing to commit to study restrictions not limited to abstinence from masturbation and sexual intercourse for specific periods.
Exclusion criteria
Patients who have aspermia, azoospermia, and cryptozoospermia.
Patients who have history/diagnosis of cancer.
Patients with testicular atrophy and congenital abnormalities not limited to absence of vas deferens.
Patients who are currently on or were on antioxidant and/or vitamin supplements up to 3 months prior to screening.
Patients with history of substance abuse.
Patients with diagnosis of HIV, HCV, HBV, and other sexually transmitted diseases.
Patients with known history of clinically significant, gastrointestinal, cardiovascular, hematological, hepatic, immunological, renal, respiratory, neurological abnormalities or diseases.
Patients who have undergone major surgical procedure 4 weeks prior to screening.
Patients who are on steroids, hormone therapy, antidepressants, and antipsychotics.
Patients who are mentally unable to comprehend the responsibilities and adhere to the stipulations of the protocol.
Patients who in the opinion of the investigator are deemed unfit to participate in the study.
Data Collection–Due permission was taken from competent authorities for data collection from Department of Reproductive Medicine Medical centre. Care seekers at reproductive medicine department for infertility treatment with >15% Sperm DNA Fragmentation and apparently healthy will be recruited after their informed written consent. Indian diabetes risk score will be calculated by using a validated questionnaire that comprised of family history, physical activity, age, and waist circumference and then classified as low risk, moderate risk, and high risk for having diabetes. Anthropometric measurement waist circumference will be taken using standard tools. Patients will be tested for diabetes on the basis of oral glucose tolerance test by venous blood sample. Test will be done and according to ADA 2004 criteria subjects will be classified as diabetics, prediabetics (impaired fasting sugar or impaired glucose tolerance), and normoglycemic.
Interventions–Combination of Co-Q10 (100 mg), Vitamin D3 (2000 I.U.), Selenomethionine (400 mcg), Astaxanthin (8 mg) tablet.
Implementation Process
Patients with repeated IVF failure or poor sperm parameter were sent for Sperm DNA fragmentation.
Simple random sampling was done during data collection; every third patient was counseled to participate in our study, if >15% DFI.
Every week, one to three patients were inducted for data collection, covering the required sample size in 10 months.
Patients were recruited after obtaining a written informed consent.
On first visit, patient information was taken according to the patient information sheet.
Patients were counseled to take medication containing antioxidant and vitamin (Co-Q10, vitamin D3, selenomethionine, and astaxanthin) for 3-month duration, twice-daily according to the general information sheet and fill IPCC (Investigational product compliance card).
On the next visit after 3 months, patient information is recorded, semen examination and sperm DFI were done to note any changes.
RESULTS
The results show that multinutrients such as Co-Q10, vitamin D3, astaxanthin, and selenomethionine combination in male subject with raised sperm DNA fragmentation Index decreases DFI index. Table 1 shows the age distribution and suggests that with age, DFI increases. The Ist DFI means the result before multinutrients shown in [Figures 1 and 2].


- Age wise distribution of infertile patients. DFI = DNA fragmentation index.

- Distribution of % DFI among different age group. DFI = DNA fragmentation index.
Figure 1 and Figure 2 and 2nd DFI (%) shows after multinutrient dose (Co-Q10 [100 mg], vitamin D3 [2000 I.U.], selenomethionine [400 mcg], and astaxanthin).
Table 1 shows distribution of the infertile patients in different age group. The present study includes infertile patients from the age group 20 to 40 years. The percent wise distribution of infertile patients was 31.84%, 31.92%, 31.46% from the age group 20 to 30, 30 to 40 and > 40, respectively. The maximum number of patients were from the age group 30 to 40 and > 40; hence, control patients were selected from the same age group with percentage 25.01% and 25.35%, respectively.
The [Table 2] shows the selected sample for the study who underwent the intervention. Result: p = 0.21 which is found out to be significant (p < 0.05 is significant). Conclusion: DFI values are found out to be decreased in all the age groups.

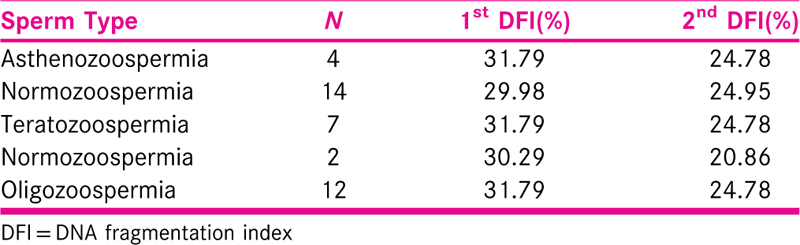
[Table 3] shows distribution of the infertile patients of Asthenozoospermia and Normozoospermia. The percent wise improvement of infertile patients with Asthenozoospermia was from 31.79% to 24.78%, from the age group 30 to 40, > 40. The percent wise improvement of infertile patients with Normozoospermia was from 29.98% to 24.95%. Result: p = 0.061 which is found out to be significant. Conclusion: DFI values are found out to be decreased in both the groups. The percent wise distribution of infertile patients with Teratozoospermia and improvement was from 31.79% to 24.78%, in the age group 30 to 40, > 40 and p = 0.36 which is found out to be significant DFI values found out to be decreased in both the groups.
DISCUSSION
Oxidative stress is the primary cause of DNA damage in spermatozoa. Both endogenous and external oxidative stress factors are discussed and many of them are quite dynamic. Antioxidants play a protective role even though oxidation and reduction must coexist in a careful balance for crucial sperm function, including fertilization. By reducing oxidative stress, a couple’s chances of conceiving—whether spontaneously or through assisted reproduction—may rise. Therefore, oxidative stress factors in men with high levels of DNA fragmentation should be looked into and diminished as needed. The improvement in male deficiency was considered as an advantage of this multinutrient rather than any negative effects being recorded as such throughout the study.
One-hundred-and-nineteen men with idiopathic and unexplained male infertility were included in a trial by Arafa et al.[18] (29 patients). A course of a combination supplement including 200 mg of Co-Q10 and other antioxidants was given to the 148 individuals. After 3 months of therapy, all semen parameters and Sperm DNA fragmentation (SDF) improved in the first group (except sperm volume and viability). SDF, however, remained constant in the group of women with unexplained infertility.
Alahmar et al.[8] looked at 50 fertile men (controls) and 50 patients with idiopathic oligoasthenoteratospermia (iOAT) who were given a daily dose of 200 mg of Co-Q10. After treatment, they discovered that SDF levels in infertile males had significantly decreased (38.6% ± 7.9 vs. 34.5% ± 9.3, p < 0.001).
Due to Co-Q10’s beneficial impact on sperm concentration and motility, some authors have reported an increase in conception rates following its treatment. A combination antioxidant treatment (CoQ, Vitamin C, and Vitamin E) enhanced the semen parameters. Kobori et al.[19] reported spontaneous pregnancies and significant improvement in sperm cell concentration.
The percent wise distribution of infertile patients[20] with Asthenozoospermia was 31.79%, 24.78%, from the age group 20 to 30, 30 to 40, >40 respectively. The percent wise distribution of infertile patients with Normozoospermia was 29.98%, 24.95%, from the age group 20 to 30, 30 to 40, >40 respectively. p = 0.061 which is found out to be nonsignificant DFI values are found out to be decreased in both the groups. The percent wise distribution of infertile patients with Teratozoospermia was 31.79%, 24.78%, from the age group 20 to 30, 30 to 40, >40 respectively. The percent wise distribution of infertile patients with Normozoospermia was 30.29%, 20.86%, from the age group 20 to 30, 30 to 40, >40 respectively. p = 0.36 which is found out to be significant DFI values found out to be decreased in both the groups. The percent wise distribution of infertile patients with Oligozoospermia was 31.79%, 24.78%, from the age group 20 to 30, 30 to 40, >40 respectively. The percent wise distribution of infertile patients with Normozoospermia was 31.6%, 25.19%, from the age group 20 to 30, 30 to 40, >40 respectively. p = 0.08 which is found out to be nonsignificant DFI values are found out to be decreased in both the groups. The percent wise distribution of infertile patients with chronic illness was 25.18%, 19.79%, from the age group 20 to 30, 30 to 40, >40 respectively. The percent wise distribution of infertile patients with chronic illness were 31.79%, 24.78%, from the age group 20 to 30, 30to 40, >40 respectively. p = 0.061 which is found out to be nonsignificant. The percent wise distribution of infertile patients with Social were 31.5%, 25.27%, from the age group 20 to 30, 30 to 40, >40 respectively. In all the groups, that is, taking alcohol, not taking alcohol or taking alcohol socially, the values of DFI are decreased. The percent wise distribution of infertile patients with Nonsmoker were 30.87%, 24.53%, from the age group 20 to 30, 30 to 40, >40 respectively. The percent wise distribution of infertile patients with Smoker was 31.79%, 24.78%, from the age group 20 to 30, 30 to 40, >40 respectively. p = 0.084 which is found out to be nonsignificant in all the groups, that is, previous smoker, nonsmoker, smoker, the value of DFI is found out to be decreased. The infertile patients with different walking age group were 30.63%, 25.23%, from the age group 20 to 30, 30 to 40, >40 respectively. The percent wise distribution of infertile patients p = 0.18 which is found out to be nonsignificant for people who are walking.[21] Hence, No Significant Difference Found In The Type Of Lifestyle Either Sedentary Or Non-Sedenta Impact of multinutrient is significant in all the groups.
CONCLUSION
It has been demonstrated that DNA fragmentation is a more reliable indication of reproductive potential than traditional semen measurements. Men who have substantial DNA fragmentation will have much lower chances of getting pregnant naturally or with IVF or techniques like intrauterine insemination. In this group, intracytoplasmic sperm injection (ICSI) may be significantly more effective and couples may be advised to move forward directly with ICSI in order to save money, prevent repeated failures, or lose pregnancies.[22,23]
The DFI in infertile patients after the COQ-10 dose was found to be significant.
Financial support and sponsorship
Nil.
Conflicts of interest
The author declares no conflict of interest.
Commentary
Male infertility contributes to approximately 40–50% of cases of infertility. The causative factors are many but in India in addition to routine causes, lifestyle and environmental stressors play a very important role in increasing oxidative stress and DNA damage. Even though new systems like MiOXSYS have been introduced, they are not commonly available and hence Sperm DNA Fragmentation is usually measured by structural assays such as Sperm Chromatin Assay.
Given the fact that increased DNA Fragmentation can cause decreased fertility rates and implantation rates or can cause recurrent implantation failure, thus influencing the outcome of IUI and IVF cycles, it makes a lot of sense to explore the beneficial effects of available oral antioxidants in potentially reversing the oxidative stress induced DNA damage.
The limitations of the above study are small sample size and the inability to use a more definitive method to determine Oxidation Reduction Potential. Also, the study does not explore the reproductive outcomes in these patients. However, the study highlights the beneficial effects of antioxidants, in this case CO-Q10, Vitamin D3, Selenomethionine, and Astaxanthin in the reduction of sperm DFI. This study could potentially form the basis of larger well designed studies with similar objectives.
As it is amply clear that poor lifestyle is often an incriminating factor in oxidative stress, it will be very prudent to couple antioxidants with lifestyle changes in studies of this kind.
Dr Venugopal, Consultant Reproductive Medicine specialist ARMC Thrissur, Visiting Consultant ARMC Palakkad, Kerala
REFERENCES
- Diagnosis and treatment of infertility in men: AUA/ ASRMAUA/ASRM Guideline Part I Guideline. J Urol. 2021;205:36-45.
- [Google Scholar]
- Sperm DNA fragmentation: A new guideline for clinicians. World J Mens Health. 2020;38:412-71.
- [Google Scholar]
- Antioxidant-based therapies in male infertility: Do we have sufficient evidence supporting their effectiveness? Antioxidants. 2021;10:1-30.
- [Google Scholar]
- Male infertility: An overview of causes and treatment options. Br J Nurs. 2016;25:35-40.
- [Google Scholar]
- The impact of two doses of coenzyme Q10 on semen parameters and antioxidant status in men with idiopathic oligoasthenoteratozoospermia. Clin Exp Reprod Med. 2019;46:112-8.
- [Google Scholar]
- The impact of two doses of coenzyme Q10 on semen parameters and antioxidant status in men with idiopathic oligoasthenoteratozoospermia. Clin Exp Reprod Med. 2019;46:112-8.
- [Google Scholar]
- Coenzyme Q10 and male infertility: A meta-analysis. J Assist Reprod Genet. 2013;30:1147-56.
- [Google Scholar]
- Coenzyme Q10, oxidative stress markers, and sperm DNA damage in men with idiopathic oligoasthenoteratospermia. Clin Exp Reprod Med. 2021;48:150-5.
- [Google Scholar]
- Conservative nonhormonal options for the treatment of male infertility: Antibiotics, anti-inflammatory drugs, and antioxidants. Biomed Res Int. 2017;2017:4650182.
- [Google Scholar]
- Vitamin D and male fertility: An updated review. World J Mens Health. 2020;38:164-77.
- [Google Scholar]
- Reactive oxygen species and male reproductive hormones. Reprod Biol and Endocrinol. 2018;16:87.
- [Google Scholar]
- Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod Biol Endocrinol. 2018;16:115.
- [Google Scholar]
- Associations of sperm DNA fragmentation with lifestyle factors and semen parameters of Saudi men and its impact on ICSI outcome. Reprod Biol Endocrinol. 2018;16:87.
- [Google Scholar]
- Oxidation-reduction potential and sperm DNA fragmentation, and their associations with sperm morphological anomalies amongst fertile and infertile men. Arab J Urol. 2018;16:87-95.
- [Google Scholar]
- Males with low serum levels of vitamin D have lower pregnancy rates when ovulation induction and timed intercourse are used as a treatment for infertile couples: Results from a pilot study. Reprod Biol Endocrinol. 2015;13:127.
- [Google Scholar]
- Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod Biol Endocrinol. 2014;12:33.
- [Google Scholar]
- Efficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. J Assist Reprod Genet. 2014;31:1161-66.
- [Google Scholar]
- Efficacy of antioxidant supplementation on conventional and advanced sperm function tests in patients with idiopathic male infertility. Antioxidants. 2020;9:219.
- [Google Scholar]
- Antioxidant cosupplementation therapy with vitamin C, vitamin E, and coenzyme Q10 in patients with oligoasthenozoospermia. Arch Ital Urol Androl. 2014;86:1-4.
- [Google Scholar]
- Predictors of pregnancy and time to pregnancy in infertile men with idiopathic oligoasthenospermia pre- and post-coenzyme Q10 therapy. Andrologia. 2022;54:e14385.
- [Google Scholar]
- Coenzyme Q10 in quail nutrition: effects on growth performance, meat quality, and myostatin gene expression. Livest Sci. 2021;252:104682.
- [Google Scholar]
- What should be done for men with sperm DNA fragmentation? Clin Exp Reprod Med. 2018;45:101-09.
- [Google Scholar]
- Supplementation of coenzyme Q10 and α-tocopherol lowers glycated hemoglobin level and lipid peroxidation in pancreas of diabetic rats. Nutr Res. 2008;28:113-21.
- [Google Scholar]







