Translate this page into:
Hyperandrogenism − approach and management
Address for correspondence: Dr. Nihita Pandey, MS (OBG), Senior Resident, Department of Obstetrics and Gynaecology, Lady Hardinge Medical College and SSKH, New Delhi, India. E-mail: kneeheeta@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Hyperandrogenism accounts for a number of distressing symptoms in the patient. Evaluation of the underlying cause is the key to the correct management of this common endocrine disorder. In this review, we analyse hyperandrogenism in terms of pathophysiology, evaluation and management options for the patient. Various Pubmed studies spanning multiple decades have been studied and evaluated in order to formulate an algorithm for diagnosis of the causes of hyperandrogenism and also to devise a management protocol for the same. The myriad underlying causes of hyperandrogenism make it imperative to have a detailed understanding of its pathophysiology so as to more effectively treat it.
Keywords
Hirsutism
hyperandrogenism
PCOS
INTRODUCTION
Hyperandrogenism is a common endocrine disorder in women, affecting around 7% of the reproductive age group and accounts for much distress in lieu of the symptoms associated.[1] Excess androgen levels in the body are responsible for a spectrum of symptoms such as hirsutism, acne, androgenic alopecia, ovulatory dysfunction, menstrual irregularity, infertility and virilisation or masculinisation being prolonged or severe.[2] The association between masculinisation and endocrine pathology was first established by Bullock and Sequera in 1905.[3] The most common cause of hyperandrogenism worldwide is polycystic ovarian syndrome (PCOS). Apart from this, in hyperandrogenic states, there can be a dysfunctional production of androgen or inadequate conversion to oestrogen or both. In this review, we aim to discuss the various causes, presentation and management options for hyperandrogenism.
ANDROGEN PRODUCTION IN FEMALES
There are two main sources of androgen production in females − that is the adrenals and the ovaries[4] [Figure 1].

- Principal sources of androgens in a normal female.
Adrenals
The adrenal glands are predominantly responsible for the synthesis and secretion of dehydroepiandosterone (DHEA) and DHEA-sulfate (DHEA-S) 90%, androstenedione 50% and testosterone 25%.
Ovaries
Ovaries produce 50% of androstenedione, 25% of circulating testosterone and about 10% to 20% of DHEA. Rest of the testosterone (50%) is produced in extra-glandular tissue by circulating DHEA and androstenedione.
ANDROGEN METABOLISM
In healthy reproductive age group women, 80% of all circulating testosterone is bound to SHBG, 19% to albumin and only 1% circulates freely. Out of the circulating androgens, only active metabolite of testosterone that is dihydrotestosterone is able to act on the androgen receptors. The remaining androgens DHEA, DHEA-S and androstenedione are bound to albumin and are peripherally converted to testosterone.[5] The circulating testosterone is metabolised in the liver into androsterone and etiocholanolone, which is conjugated with glucuronic acid or sulfuric acid and excreted in the urine.
CAUSES OF HYPERANDROGENISM
Idiopathic
Idiopathic hirsutism occurs more frequently in certain ethnic population, due to skewing of X chromosome carrying the androgen receptor gene. This causes increased androgen receptor-mediated sensitivity of the hair follicle.[6]
Polycystic ovarian syndrome
PCOS is a syndrome complex that comprises of anovulation and raised androgen levels. It is manifested by hirsutism, menstrual abnormality like oligomenorrhoea, amenorrhoea, dysfunctional uterine bleeding or obesity. It affects around 10% to 20% of the reproductive age population. The basic pathology lies in dysregulation of enzyme cytochrome P450-17-∝ which is present in ovaries and adrenals and catalyses the activities of two enzyme systems that is 17-hydroxylase and 17,20 lyase resulting in hyperandrogenism.[7] PCOS is also associated with hyperinsulinemia and insulin resistance that leads to an increase in ovarian androgen production. In the liver, it reduces the production of insulin growth factor-binding protein-I and SHBG, which further increases free androgen levels.
Stromal hyperthecosis
Hyperthecosis is a result of the differentiation of ovarian interstitial cells into testosterone producing luteinised stromal cells.[8] These patients generally have normal LH and DHEAS levels but a higher serum insulin and testosterone level than those with PCOS. Patients with hyperthecosis present with signs of virilisation and decreased feminisation. The diagnosis can be confirmed only by histologic examination of the ovaries, which reveal nests of luteinised cells in ovarian stroma.
Congenital adrenal hyperplasia (CAH)
It can present in three forms[9]:
-
Autosomal recessive: It is severe, presents soon after birth with evidence of adrenal insufficiency and ambiguous genitalia. There is deficiency of 21-hydroxylase leading to accumulation of 17-hydroxy progesterone which converts to excess androgens and deficiency of cortisol and aldosterone [Figure 2].
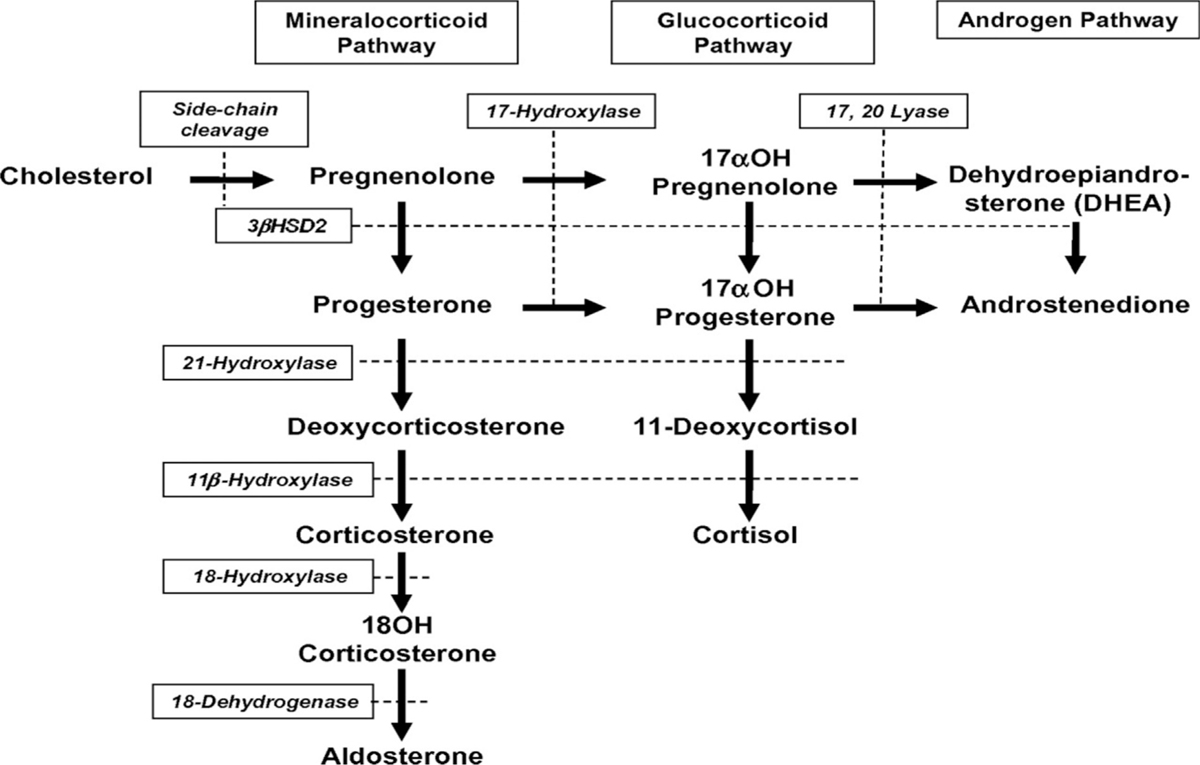 Figure 2
Figure 2- Androgen synthesis pathway.
Late onset: Slowly progressive, detected in early adulthood with short stature, irregular menstruation and positive family history. Onset of hirsutism is at puberty and occurs due to partial deficiency in the activity of 21-hydroxylase.
Cryptic form: Generally detected by biochemistry with the patient presenting with history similar to PCOS and have hyperandrogenic chronic anovulation.
The diagnosis of late-onset 21-hydroxylase deficiency requires an elevation of early morning 17-hydroxy progesterone in the follicular phase.
Hyperinsulinemia
Insulin resistance can also lead to hyperandrogenism, although it is not a mandatory outcome. Various practical criteria have been suggested for the diagnosis of insulin resistance such as body mass index >27 kg/m2, a waist/hip ratio >0.85, the presence of acanthosis nigricans, impaired glucose tolerance, an elevated fasting or postprandial insulin concentration and fasting glucose/insulin ratio <4.5. Hyperandrogenism associated with insulin resistance and acanthosis nigricans come under the entity of hyperandrogenism insulin resistance and acanthosis nigricans (HAIR-AN) syndrome.[10,11]
Rare causes
Rare causes of hyperandrogenism include virilising ovarian tumours and adrenal tumours. They are associated with rapid virilisation and progression of symptoms. Other rare causes include hyperprolactinoma that leads to hirsutism secondary to chronic anovulation. Cushing’s syndrome and gonadal dysgenesis may also present with signs of hyperandrogenism.
CLINICAL PRESENTATION OF HYPERANDROGENISM
Acne
Persistence of acne into later teens should alert the endocrinologist to the possibility of hyperandrogenism especially if it is associated with hirsutism or menstrual irregularity.[12,13] Excess sebum production due to androgens leads to blockage of glands or pores, wherein bacteria grow and multiply leading to inflammation.
Androgenic alopecia
Androgenic alopecia, which is hair loss caused by male hormones, tends to affect the temples, the crown and the vertex. This is due to stimulation of hair growth elsewhere thereby retarding scalp growth. About 15% of women in reproductive age group presenting with alopecia and no other signs of hyperandrogenism have hyperandrogenaemia.[14]
Acanthosis nigricans
This occurs in severe hyperandrogenism and hyperinsulinemia. It presents as a velvety, mossy verrucous, hyperpigmented skin change over the nape of the neck, axillae, beneath the breast and in other body folds. Histologically, it represents hyperkeratosis, epidermal papillomatosis and hyperpigmentation.
Hirsutism
Hirsutism is defined as the presence of hair in locations where hair is not commonly found in women. These hairs are of dark colour, coarse texture and occur in androgen-dependent areas particularly over the upper lip, breast, chest, intermammary region, inner thigh and lower back.[15,16]
Depending on the effect of androgens on various types of hair, these can be divided into three groups:
Hair that show no androgen dependence, for example lanugo, eyebrows and eyelashes.
Hair dependent on adrenal androgens adrenocorticotropic hormone (ACTH) such as axillary or pubic hair.
Hair dependent on gonadal androgens luteinizing hormone (LH) such as midline, facial and intermammary except scalp hair.
Hypertrichosis
It is a term reserved for androgen-independent growth of hair that is seen in nonsexual areas like trunk and extremities.
Virilism
It occurs because of severe hyperandrogenism and is characterized by temporal balding, deepening of voice, decreased breast size, increased muscle mass, loss of female body contour, clitoral enlargement and amenorrhoea. It is usually seen in androgenic tumours.
DIAGNOSTIC WORKUP OF A PATIENT WITH HYPERANDROGENIC DISORDER
History
A detailed history pertaining to the patient’s lineage needs to be taken. Other important details are as follows:
Age of onset: during childhood it could be due to CAH. During puberty it could be due to PCOS, CAH and Stein-Leventhal syndrome.
Mode of onset: it is rapid in ovarian or adrenal tumours whereas it is slow, progressive in CAH and PCOS.
Personal history: a stressful event in life such as change of place, occupation or marriage can cause disturbance in hypothalamic pituitary axis.
Menstrual disorders: generally present with oligomenorrhea in PCOS, although women with gonadal dysgenesis and virilising tumours may present with amenorrhoea.
Family history: history in other family members can point towards genetic predisposition, PCOS, metabolic syndrome and CAH.
Drugs history: intake of androgens, danazol, 19-norprogesterone and minoxidil.
Clinical evaluation
Height, weight and body mass index should be measured since a growth spurt can be seen in CAH or virilising tumours, and women with polycystic ovarian syndrome (PCOS) and metabolic syndrome can present with increased weight.
Blood pressure may be high in patients with Cushing’s syndrome, PCOS and metabolic syndrome.
An examination of the thyroid should also be carried out.
Abdomino-pelvic examination is not very reliable for ruling out neoplasms, as these tumours are small and patients are usually obese. Ovaries may be palpable in PCOS.
Clitoromegaly may be present in patients of CAH, virilising tumours and a few cases of PCOS. Skin evaluation can detect hyperpigmentation and acanthosis nigricans in case of HAIR-AN syndrome or Cushing’s syndrome.
Clinical assessment of hirsutism should be made using the Ferriman-Gallway score. A score of 7 or less is normal, 8–15 suggests mild hirsutism and more than 15 suggests severe hirsutism.
Figure 3 gives a pictorial representation of the score.
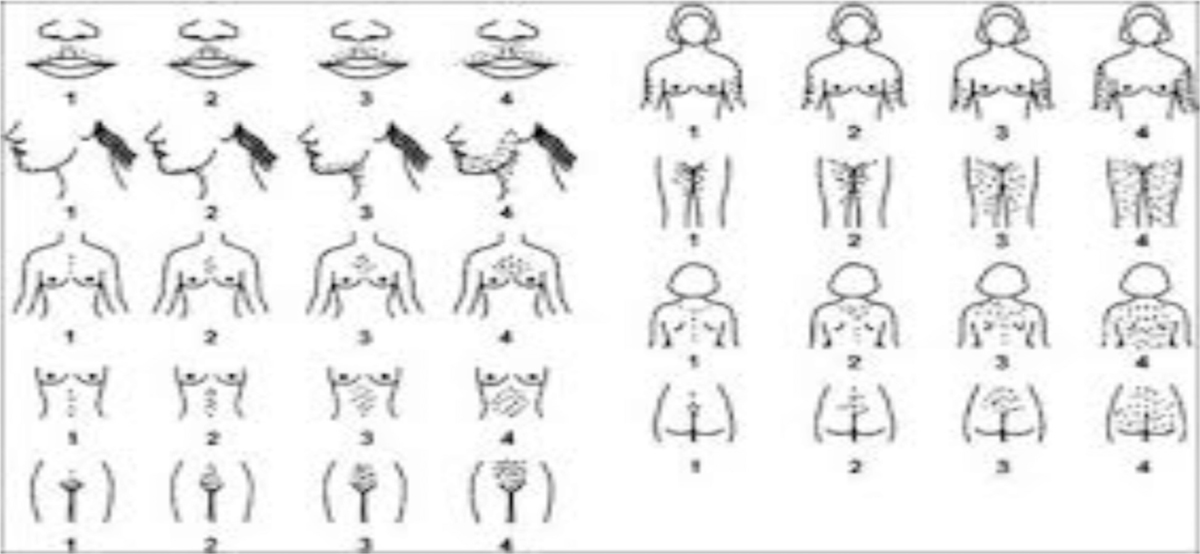
- Ferriman Gallway scoring
Investigations
The algorithm of evaluation of a woman presenting with features of hyperandrogenism has been summarised in Figure 4.[17,18,19,20]
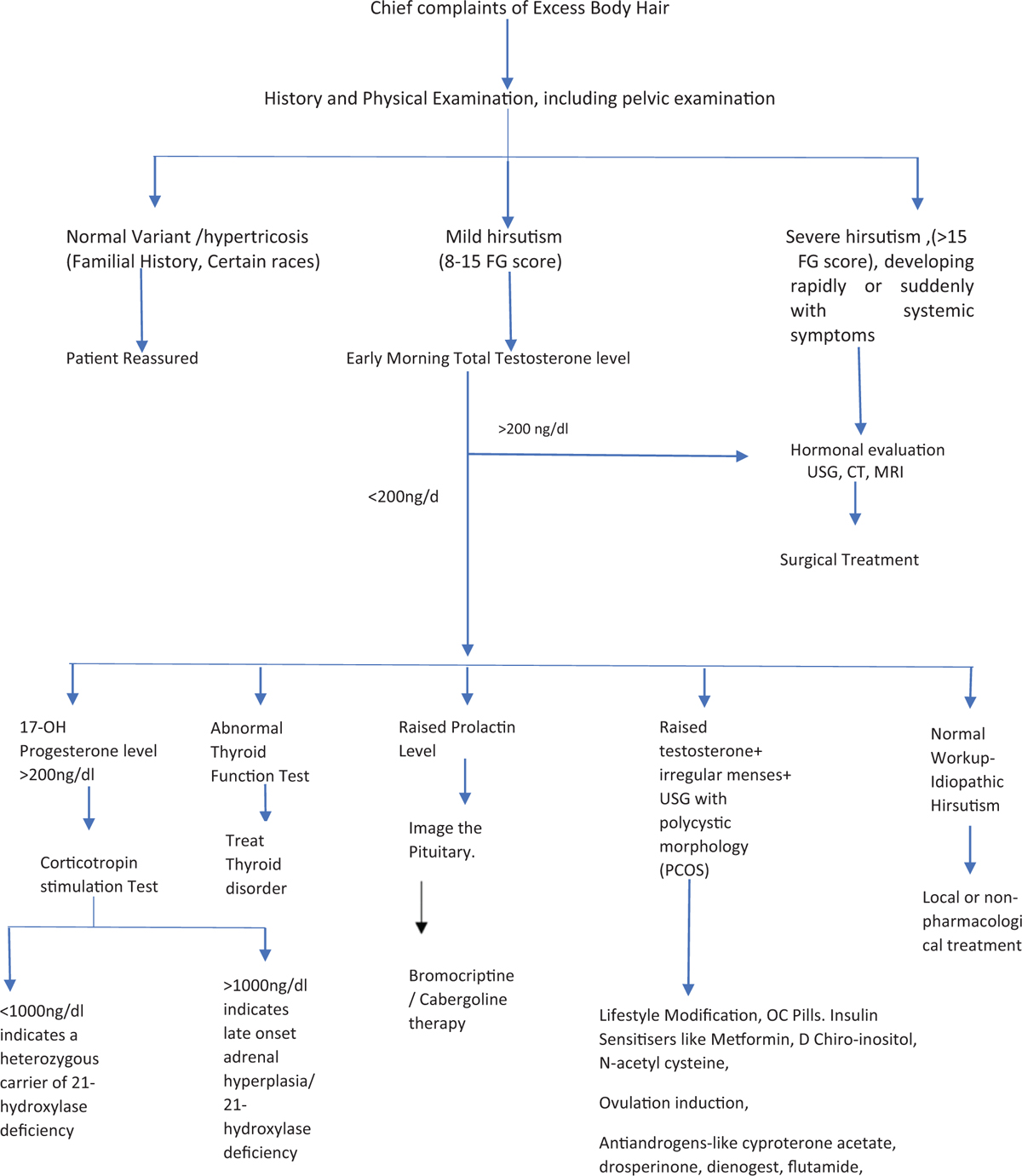
- Algorithm of evaluation of a woman presenting with features of hyperandrogenism.
Management revolves around identification and treatment of the underlying cause. The various modalities of treatment are summarized in Table 1.
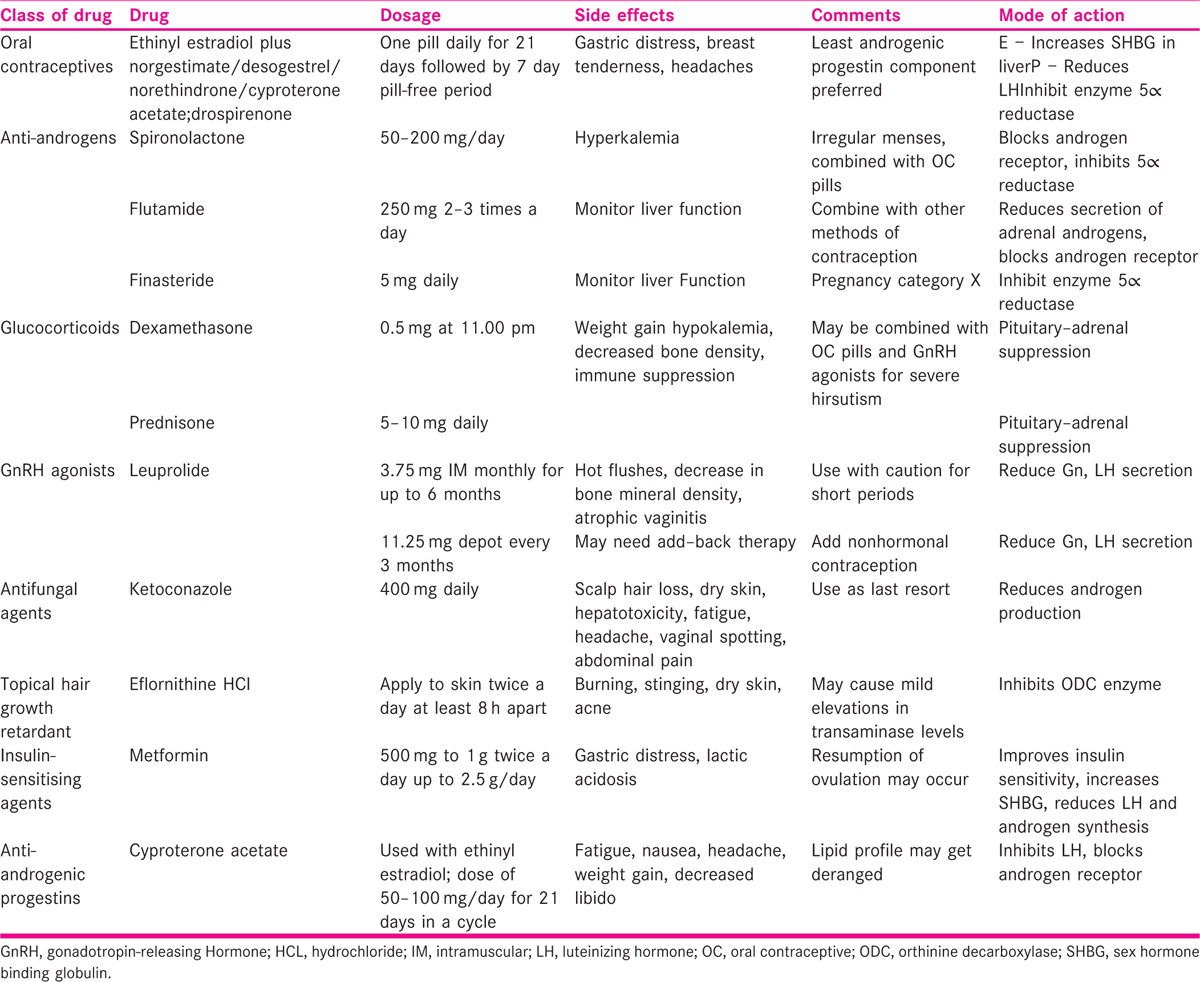
Other non-pharmacological methods for hair removal can be used such as epilation, shaving, plucking, waxing, depilation and some permanent methods such as thermolysis, electrolysis and laser treatment.
CONCLUSION
Hyperandrogenism is a complex problem with varied manifestations. This review gives an outline of a scientific approach to a patient presenting with different clinical manifestations of hyperandrogenism. The treating physician must determine the cause of excess androgens and look for any evidence of damage to other organs. Treatment should be aimed to target the specific aetiology with the aim of prevention of long-term development of metabolic syndrome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Polycystic ovary syndrome and the differential diagnosis of hyperandrogenism. Obstetr Gynaecol. 2013;15:171-6.
- [Google Scholar]
- Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078-82.
- [Google Scholar]
- The relation of the suprarenal capsules to the sexual organs. Trans Path Soc Lond. 1905;56:189-208.
- [Google Scholar]
- Diagnosis of hyperandrogenism: biochemical criteria. Best Pract Res Clin Endocrinol Metab. 2006;20:177-91.
- [Google Scholar]
- The climacteric ovary as a functional gonadotropin-driven androgen-producing gland. Fertil Steril. 1994;62:20-7.
- [Google Scholar]
- Polycystic ovary syndrome: clinical perspectives and management. Obstet Gynecol Surv. 1999;54:403-13.
- [Google Scholar]
- Treatment of severe androgen excess due to ovarian hyperthecosis with a long-acting gonadotropin-releasing hormone agonist. Am J Obstet Gynecol. 1986;154:1241-8.
- [Google Scholar]
- Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;21:245-91.
- [Google Scholar]
- Clinical profiles, occurrence, and management of adolescent patients with HAIR-AN syndrome. Sci World J. 2004;4:507-11.
- [Google Scholar]
- An update on polycystic ovary syndrome − its investigation and management: some researchable issues. Health Populat Persp Issues. 2004;27:126-66.
- [Google Scholar]
- Pathomechanism of androgenetic alopecia and new treatment. Nippon Ronen Igakkai Zasshi. 2004;41:598-600.
- [Google Scholar]
- Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440-7.
- [Google Scholar]
- Utilization of commercial laboratory results in management of hyperandrogenism in women. Endocr Pract. 1998;4:1-10.
- [Google Scholar]
- The prognostic value of acute adrenal suppression and stimulation tests in hyperandrogenic women. Fertil Steril. 1982;37:187-92.
- [Google Scholar]
- ACTH stimulation tests and plasma dehydroepiandrosterone sulfate levels in women with hirsutism. N Engl J Med. 1990;323:849-54.
- [Google Scholar]







