Translate this page into:
Role of endometrial receptivity array in recurrent implantation failure
Address for correspondence: Dr Samadhiya Richa, Department of Reproductive Medicine, Bansal Hospital, Bhopal, Madhya Pradesh, India. E-mail: richa.dr17@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
The endometrial receptivity array (ERA), a customized microarray, is an objective test to assess the endometrial receptivity status of infertile patients. It provides an opportunity to do personalized embryo transfer (pET) by synchronizing with each patient’s window of implantation (WOI) thereby increasing the success of treatment particularly in couples with recurrent implantation failure (RIF).
Aim:
To find out whether pET after ERA testing in couples with RIF improves implantation and pregnancy rates.
Materials and methods:
This is a retrospective analysis of women with a history of RIF undergoing further infertility treatment at our center. In this study, records of 34 women with history of RIF who consented to undergo ERA from July 2016 to July 2020 were analyzed.
Results:
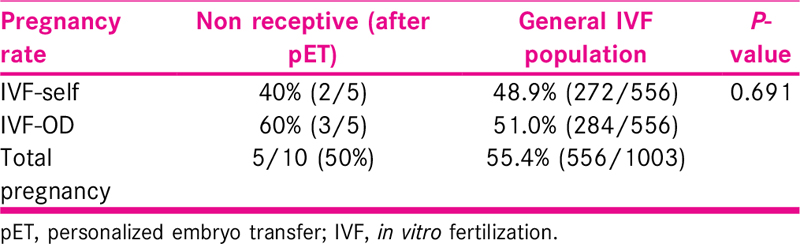
Thirty-four women with RIF who underwent ERA testing showed receptive endometrium in 21 patients (61.8%) and nonreceptive endometrium in 13 (38.2%) patients. Of these, 27 patients were included for analysis and they underwent total of 31 embryo transfer cycles. ERA showed receptive endometrium in 17 patients and nonreceptive in 10 patients. Among eight women who had nonreceptive ERA result which means displaced WOI pET resulted in an implantation rate of 45.5% and pregnancy rate of 50% which was comparable to the 55.4% background pregnancy rate of the general in vitro fertilization population during the same time period.
Conclusion:
Hence, there is a subset of patients with RIF who can achieve a pregnancy rate as good as general population with pET based on ERA results and ERA can be recommended to such patients.
Keywords
ERA
pET
recurrent implantation failure
window of implantation
INTRODUCTION
Assisted reproductive technology (ART) has allowed many couples who were previously unable to conceive to attain a viable pregnancy.[1] Despite various breakthrough achievements in history of reproductive medicine, endometrium has long been neglected and considered as a passive part of the process of implantation with the primary focus being the embryo. Beginning from morphologic assessment to time-lapse, plenty of research remained focused on finding a good-quality embryo.
After so much refinement of embryo quality and embryo transfer techniques since the inception of ART attainment of live-birth rates of only 25% to 30% per started cycle suggests that something is still missing in the evaluation and workup of infertile couples.[2] Particularly couples with recurrent implantation failure (RIF) who have been transferred good-quality euploid embryos are quite puzzled about cause of their cycle failure. Multiple failed cycles can leave couples devastated and often furious.
The process of implantation in humans occurs in a narrow time frame and involves a complex interaction between a blastocyst and endometrium. During a natural cycle in a women, the embryo enters the uterine cavity ∼4 days after ovulation.[3] The endometrium becomes receptive to implantation of blastocyst 6 to 8 days after ovulation and remains so for the next ∼4 days (cycle days 20–24).[4] In ART cycles, this process is artificially mimicked through administration of sequential estrogen and progesterone.
Implantation failure may be because of embryo or endometrial factors. Failure of the endometrium to attain receptivity is one of the causes of infertility and RIF and this is not being currently assessed during workup of infertility due to lack of credential markers for receptivity. RIF has been defined as failure to achieve a clinical pregnancy after transfer of at least four good-quality embryos (on the basis of morphologic assessment) in a minimum of three fresh or frozen cycles in a woman <40 years of age.[5] In about one-third of embryo transfers, even euploid morphologically normal blastocysts fail to implant which suggest that a nonembryonic cause probably a change in endometrial receptivity may be held responsible for implantation failure.[6,7]
One of the changes in receptivity might involve the shift in timing of the window of implantation (WOI), previously thought to be the same among all women. The WOI lasts 30 to 36 hours and depending on the patient, occurs between LH+6 to LH+9 in natural cycles or from P+4 to P+7 in hormonal replacement therapy (HRT) cycles.[8]
Traditionally, the means of monitoring of the WOI is by transvaginal ultrasonography and blood hormone levels, but these parameters lack accuracy and objectivity and neither is able to predict the pregnancy outcomes.[9]
Owing to the short window of opportunity for blastocysts to implant in the human endometrium, the embryo transfer day is carefully selected such that the endometrium is in temporal synchrony with the developmental stage of the embryo. To achieve this target, a objective method to identify WOI is essential especially in subgroup of women suffering from repeated in vitro fertilization (IVF) failure otherwise the decision to continue with further IVF treatment can be frustrating and difficult.[10]
The endometrial receptivity array (ERA) was the first diagnostic test developed to address the endometrial receptivity status of infertile patients. It consisted of a customized microarray containing 238 differentially expressed genes coupled to a computational predictor able to identify the transcriptomic profiles of proliferative (PRO), prereceptive (PRE), receptive (R), or postreceptive (POST) endometrial samples. The authors in their pioneering study have shown that one in four patients with RIF has a displaced/asynchronous WOI and a personalized embryo transfer (pET) resulting in a 50.0% pregnancy rate (PR) and 38.5% implantation rate (IR), similar to that of controls.[8]
The techniques employed for ERA testing has evolved over the years. In clinical practice, next generation sequencing (NGS) technology has replaced microarray and polymerase chain reaction-based clinical tests.[11] Single-cell RNA sequencing (scRNA-seq) is the most recent one in this field and the signatures revealed that in humans, WOI opens with an abrupt and discontinuous transcriptomic activation in the epithelia, along with a widespread decidualization in the stromal fibroblasts.[12]
The present study was therefore conducted to find out whether pET after ERA testing affects the IR and PR in patients with previous failed self or oocyte donation embryo transfers.
MATERIALS AND METHODS
Subjects
A retrospective analysis of women with a history of RIF undergoing further infertility treatment at our center. In this study, we examined 34 women with a history of RIF who consented to undergo ERA from July 2016 to July 2020. Women were in the age group of 28 to 50 years with average body mass index of 26.6 ± 4.3 kg/m2.
Inclusion and exclusion criteria
Patients with at least two previous IVF failures with morphologically good-quality embryos who consented for ERA were included for the analysis, whereas patients with uncorrected uterine and adnexal pathologies were excluded. Apart from routine infertility workup a hysteroscopy/3D TVS, thyroid function tests, and couple karyotyping were carried out in all patients.
Endometrial sampling and processing
All the women underwent an endometrial biopsy for the ERA test in a HRT. Endometrium was prepared using oral estrogen in a dose of 6 mg per day (estradiol valerate 2 mg, tablet Evadiol, Intas Pharmaceuticals Limited, Gujarat, India) which was started on day 1/2 of the menstrual cycle after a baseline transvaginal ultrasound. On day 12/13, ultrasound was repeated to measure endometrial thickness and confirmed ovarian suppression. After an appropriate endometrial thickness of >7 mm was achieved, progesterone was started in the form of vaginal progesterone gel (Naturogest gel 8%, Zydus Healthcare Limited, Ahmedabad, India) once a day and injection Sugest 100 mg IM (micronized progesterone) every alternate day, and on day P+5, an endometrial biopsy was performed from the uterine cavity with the use of Pipelle catheter (Laboratoire CCD, Paris, France). Endometrial tissue was transferred to a cryotube containing 1.5 mL RNA stabilizing agent (Qiagen India Pvt. Ltd.) and then shaken vigorously for 10 seconds, and kept at 4°C in refrigerator for 4 hours. Caution was exercised while transferring the endometrial tissue into cryotube so that the amount of tissue did not exceeded the white line on the cryotube.[13] The samples were transported at room temperature to Igenomix India (New Delhi, India). The test results were available in 2 weeks.
ERA interpretation
The ERA is a molecular diagnostic method that uses NGS to simultaneously measure the expression profile of 248 genes of endometrial cells that have been previously identified as the transcriptomic signature of endometrial receptivity and a bioinformatics tool (predictor) that gives a diagnosis with a specific diagnostic probability.[13] ERA test diagnosed the endometrium to be receptive (R) or nonreceptive (NR). NR was further classified as pre- or postreceptive. Patients with the receptive endometrium (P+5) underwent frozen embryo transfer (FET) in a subsequent HRT cycle simulating the ERA cycle. In patients with a changed WOI, FET was performed in the pWOI on the basis of ERA test results. Two good-quality embryos were transferred.
Statistical methods
For continuous data, the descriptive statistics was used such as mean and standard deviation. Frequency and percentage were used for categorical data. To assess the association between two categorical variables, the Fisher exact test was used. All tests were two-sided at α = 0.05 level of significance. Data were entered using Microsoft Office Excel 97-2003 worksheet.
RESULTS
Results of ERA
In our study, ERA test results were documented for 34 patients with a history of RIF. ERA showed receptive endometrium in 21 patients (61.8%) and nonreceptive endometrium in 13 (38.2%) patients. Of these, 27 patients were included for analysis as 7 patients had not underwent embryo transfer till the date of data collection and 1 patient had fertilization failure. Twenty-seven patients underwent total of 31 embryo transfer cycles. ERA showed receptive endometrium in 17 patients and nonreceptive in 10 patients as mentioned in Table 1. A total of 17 embryo transfers were carried out in samples showing receptive ERA result, 13 were self and 4 were donor cycles. PR achieved was 47.05% and IR was 23.2%.clinical PR and live-birth rate was 47.05% and 46.66%, respectively, as mentioned in Table 2.


Among nonreceptive endometrium, eight patients had a prereceptive endometrium and only two patients had postreceptive result. In these patients, in subsequent cycles, ERA was performed to find out the personalized window and FET was performed accordingly. A total of 10 embryo transfers were carried out including 5 self and 5 donor cycles. PR achieved was 50%. IR was 45.5%. Clinical PR and live-birth rate achieved after pET were 40% and 30%, respectively, as shown in Table 3.

When comparison was carried out between receptive and non receptive ERA results, pregnancy outcomes were not statistically significant, as shown in Table 4. This may be due to small sample size.

Our results show that in women with RIF, there is a subset of patients with displaced WOI who can achieve a PR as good as general IVF population with pET based on ERA results, as shown in Table 5. PRs in general IVF population was 48.9% in self cycles and 51% in donor cycles which is comparable to personalized transfer after ERA in nonreceptive endometrium.
DISCUSSION
Endometrial receptivity at the time of WOI is a crucial moment of the menstrual cycle, and its understanding has been one of the main goals for researchers working in human reproduction. RIF is an important clinical entity with still no universally established criteria. The incidence of RIF is unknown, and the true significance of numerous factors that have been implicated in its pathogenesis remains to be determined.
Individualized protocols are being promoted to optimize treatment in various fields including ovarian stimulation. Approach for individualized embryo transfer is particularly beneficial for subgroup of patients with RIF.
In our study, we found that in women presenting with two or more implantation failures about 37.03% showed a displaced WOI. Hashimoto et al. in 2017 studied 50 patients with RIF and found that 24% of RIF patients had displaced WOI.[14] Ruiz-Alonso et al. in 2013 suggested a displaced WOI in 27.5% patients.[8] Mahajan in 2015 studied 186 infertile women including women with RIF and thin endometrium who underwent ERA and found that 27.5% women had nonreceptive endometrium in the RIF subgroup.[10]
Among patients with a displaced WOI, the majority of cases were prereceptive (84.61%), which is also consistent with previously reported studies.[8]In the present study, in the subgroup of women showing nonreceptive ERA who underwent the appropriate adjustment in timing of FET according to the ERA test conceived in about 50% of cases. This PR is comparable to the 55.4% background PR of the general IVF population during the same time period; however, the results were not significant.
Tan et al. in their retrospective review of 88 patients with at least one previously failed euploid FET found that implantation and ongoing PRs were higher after personalized ET when compared with patients without pET (73.7 vs. 54.2% and 63.2 vs. 41.7%) although differences were not statistically significant.[15]
Limitations of this study include a small sample size and lack of a control arm to compare reproductive outcomes among patients with a history of implantation failure who did not undergo pET with ERA. Also assessing embryo quality by morphology alone rather than assessing euploidy status is a drawback of the study.
Our experience shows that a significant proportion of patients with a history of implantation failure has displaced windows of implantation and may benefit from personalized adjustment in the timing of FET. Although larger randomized studies are required to validate these observations, our initial experience demonstrates that ERA may be a promising technique to help characterize endometrial receptivity and provide directives to improve implantation in instances of previous failure and nonreceptive endometrium.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The early history of IVF in Australia and its contribution to the world (1970–1990) Aust N Z J Obstet Gynaecol. 2004;44:495-501.
- [Google Scholar]
- International Committee for Monitoring Assisted Reproductive Technology: world report on assisted reproductive technology, 2011. Fertil Steril. 2018;110:1067-80.
- [Google Scholar]
- In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100:100-7.
- [Google Scholar]
- The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil Steril. 1992;58:537-42.
- [Google Scholar]
- Recurrent implantation failure: definition and management. Reprod Biomed Online. 2014;28:14-38.
- [Google Scholar]
- In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100:100-7.
- [Google Scholar]
- The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818-24.
- [Google Scholar]
- Endometrial thickness and serum oestradiol concentrations as predictors of outcome in oocyte donation. Hum Reprod Oxf Engl. 1997;12:2271-6.
- [Google Scholar]
- Endometrial receptivity array: clinical application. J Hum Reprod Sci. 2015;8:121-9.
- [Google Scholar]
- Endometrial receptivity analysis (ERA) using a next generation sequencing (NGS) predictor improves reproductive outcome in recurrent implantation failure (RIF) patients when compared to ERA arrays. In: Human Reproduction [Internet]. Oxford University Press; 2018. p. :8.
- [Google Scholar]
- Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat Med. 2020;26:1644-53. doi: 10.4103/0974-1208.165153
- [Google Scholar]
- ERA Test | Igenomix ERA IVF Biopsy for infertility treatments [Internet]. Available at https://www.igenomix.com/our-services/era/
- Efficacy of the endometrial receptivity array for repeated implantation failure in Japan: a retrospective, two-center study. Reprod Med Biol. 2017;16:290-6.
- [Google Scholar]
- The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers. J Assist Reprod Genet. 2018;35:683-92.
- [Google Scholar]







