Translate this page into:
Triggers in controlled ovarian hyperstimulation
Address for correspondence: Dr. M. Gouri Devi, 30, Malka Ganj Road, Jawahar Nagar, New Delhi 110007, India. E-mail: gouri48@ridgeivf.com
-
Received: ,
Accepted: ,
How to cite this article: Devi MG. Triggers in controlled ovarian hyperstimulation. Fertil Sci Res 2023;10:183-7.
Abstract
Controlled ovarian hyperstimulation (COH) is a crucial component of assisted reproductive technologies like intrauterine insemination (IUI) and in vitro fertilization (IVF). Triggers in COH play a pivotal role in maturation of the follicles in their final stages and optimizing the timing of egg retrieval, which is very important for the collection of maximum eggs and for a good success rate. Human chorionic gonadotropin (hCG) is used as a standard method for the final follicular maturation and ovulation. HCG has the similar effect as luteinizing hormone (LH) with a half-life of 5–7 days. Now a days, gonadotropin-releasing hormone agonist (GnRH-a) trigger has been used with the target to reduce OHSS for the induction of final follicular maturation and ovulation. Based on the results of various studies, using the GnRH-a trigger leads to defects in the luteal phase resulting in reduced implantation and clinical pregnancy rates and also increasing abortion rates in fresh embryo transfer cycles compared to the routine IVF cycle with hCG triggering. In this review, we examined the benefits, problems and also ways to reform various triggers used for ovulation.
Keywords
Hyperstimulation
ovulation
triggers
INTRODUCTION
Ovulation is the process whereby the dominant follicle once reaches the optimum size of 16–18 mm, then it ruptures, and the ovum is released near or into the Fallopian tube. In a natural cycle of 28 days, this occurs around the 14th day of the cycle. As the estrogen levels in the follicular rise and the optimum level is reached and plateaus for 48 hours, the negative feedback on the pituitary leads to positive feedback on the hypothalamus, thereby increasing the pulses of GnRH to the anterior pituitary, leading to an LH surge that result in ovulation.[1]
Material and methods
Thirty articles were studied and analysed for this study using various online sites such as PubMed, Google scholar, Scopus etc. Every article selected for the journal was evaluated based on sample size, journal, ethnicity and then selected for the review study.
DISCUSSION
Controlled ovarian hyperstimulation (COH) is a crucial component of assisted reproductive technologies like intrauterine insemination (IUI) and in vitro fertilization (IVF). Triggers in COH play a pivotal role in maturation of the follicles in their final stages and optimizing the timing of egg retrieval, which is very important for the maximum eggs collection and a good success rate.
In a natural menstrual cycle, it is the endogenous Luteinizing hormone (LH) surge that causes ovulation. Ovarian control of the gonadotropins is achieved by the negative and positive feedback mechanisms of estrogen and progesterone, but there is a cascade of events that leads to ovulation in a natural cycle. Inhibition A and B are nonsteroidal substances that influence the gonadotropin release, besides gonadotrophin surge-attenuating factor (GnSAF) which is said to play an important role in LH surge.[2] There is a neuroendocrine metabolism that involves ovarian oestradiol levels and the circadian rhythm, which leads to the LH surge. This LH surge has been replaced by human chorionic gonadotropin (HCG) for assisted reproductive cycles (ART).
There are the following triggers used in clinical practice in the assisted reproductive technology
Human chorionic gonadotropin (HCG)
The most widely used trigger in COH is HCG. The follicular rupture occurred 34–38 hours after HCG administration.[3] LH surge causes the final maturation and release of ovum in natural cycles. It is the corpus luteum, which releases the HCG that helps the luteal phase of the cycle.
The process of ovulation is a complex event that involves a series of connected steps, which lead to ovulation. There has to be the formation of an extracellular hyaluronan (HA)-rich matrix by the cumulus oocyte complex (COC), a process called expansion. HA-binding proteins and other factors like cyclogenase-2 (COX-2), proteoglycan versican (a substrate of the protease), disintegrin and metalloproteinase with thrombospondin-like repeats (ADAMTS-1-this co-localises the versican proteoglycan for ovulation) all play an important role in ovulation.[4]
The half-life of HCG is more than 24 hours, and this luteotropic activity can lead to the release of vasoactive substances like vascular endothelial growth factor (VEGF).[5] These vasoactive substances are responsible for the ovarian hyperstimulation syndrome (OHSS), especially in hyper responders, due to their direct effect on the ovarian follicles. Due to the risk of OHSS, the GnRH agonist triggers come up in the picture, which makes it possible to form OHSS-free clinics.
HCG triggers: The preparations available:
Human chorionic gonadotropin: dose 5000–10,000 I.U.
Recombinant HCG 250 mcg (6500 I.U.) dose-250–500 mcg
Gonadotropin releasing hormone agonist (GnRHa)
Forty years ago, Nakano et al. described that a single injection of GnRHa was enough to cause the LH surge to trigger the ovulation.[6] However, this was undervalued as GnRHa was used in a long protocol to prevent premature luteinisation. When the third-generation GnRH antagonist came into vogue to prevent LH surge, then GnRHa as a single bolus could be used as a trigger in place of HCG, as the antagonist action lasts only for 24 hours.[7] The normal spontaneous LH surge of the natural menstrual cycle is characterized by a short ascending phase (14 hours), a peak plateau phase (14 hours) and a long descending phase (20 hours) that is three phasic[8] and lasts for a total of 48 hours. In GnRHa-induced surge lasts only for 24 hours (Biphasic). Fig. 1
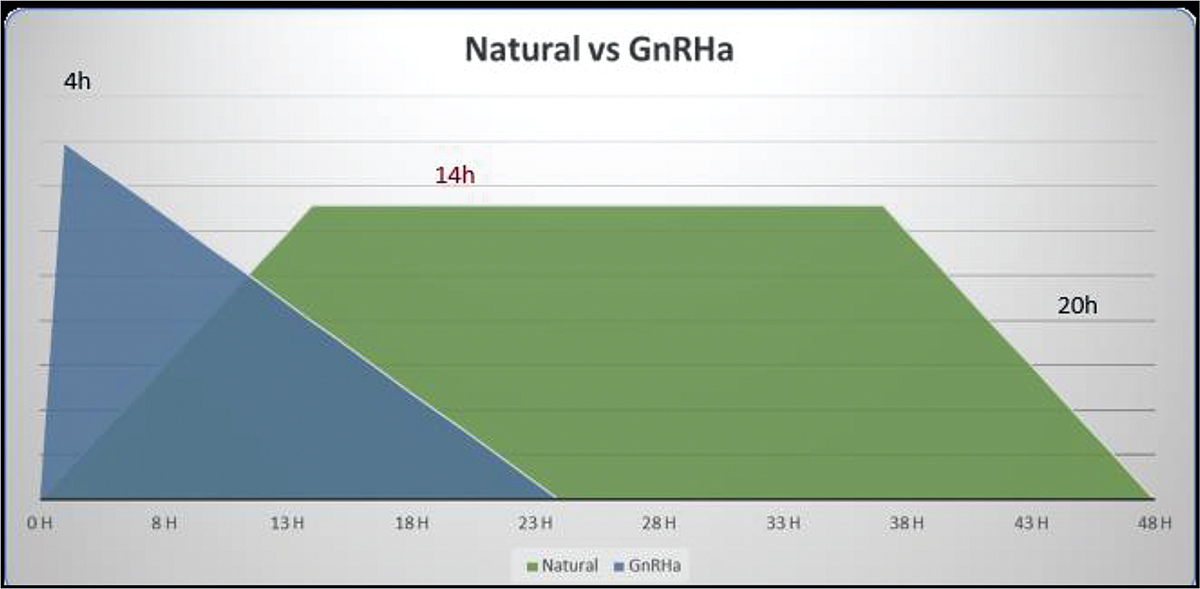
- Nautral versus GnRHa.
So GnRHa is as good as HCG in the final maturation of the oocyte and additionally prevents OHSS.[9] When the GnRHa trigger is given, there is also an FSH surge, which promotes the formation of LH receptors in luteinizing granulosa cells and seems to promote oocyte nuclear maturation and cumulus expansion.[10] But GnRHa induces a shorter duration of LH exposure, which can lead to luteal insufficiency, hence hampering the pregnancy rates. This has to be considered when giving luteal support
Following are the commonly used GnRHa:-
Recombinant LH as a trigger?
Comparative studies were done with HCG, Rec, LH, and the 15,000 IU to 30,000 IU dose of rhLH provided the highest efficacy-to-safety ratio.[14]The cost of the high dose versus efficacy has not become the default triggering option. A 2016 Cochrane analysis found that the quality of evidence regarding rLH performance was very low.[15]
Kisspeptin
Kisspeptin is a peptide hormone that has an important role in the neuroendocrine regulation of human reproduction.[16] Kisspeptins are a family of arginine phenylalanine amide (RF amide) peptides encoded by the KISS1 gene located on chromosome 1q32. Kisspeptins are potent stimulators of the hypothalamo-pituitary-gonadal axis. KP signals directly act on the KP receptor to release GnRH, which in turn stimulates the secretion of both LH and FSH from the gonadotrophs of the anterior pituitary, although the effect on the former is more marked. The ability of kisspeptin to induce an ovulatory LH rise suggested that it could be used to induce oocyte maturation in women undergoing IVF treatment.[17]
KSP-54 induces LH levels similar to natural LH surges and is comparable to other triggers. Recently, MVT-602, a kisspeptin receptor analogue, has seen to have longer pharmacodynamic action than the native peptide, KP54. MVT-602 is a nanopeptide kisspeptin receptor agonist developed through modification of KP-10 and has more stability, potency and water solubility.[18]
The different triggers and their peak blood levels have been demonstrated in Figure 2. More studies are needed with Kispeptin before it can be regularly used as a trigger on routine basis.
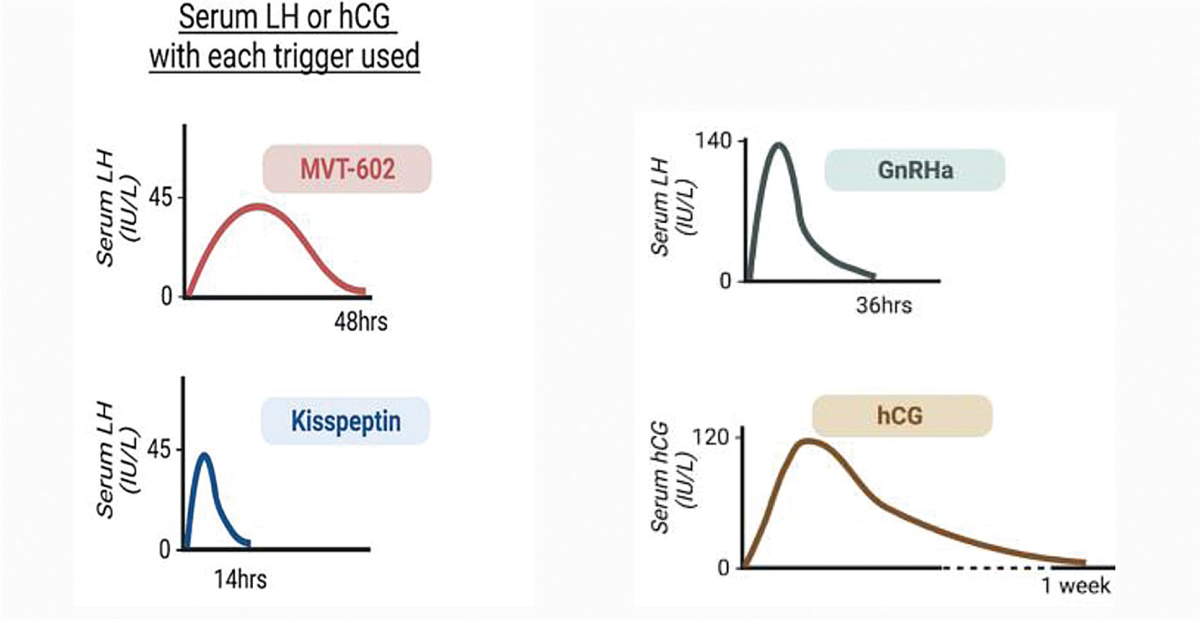
- Serum LH or hCG with each trigger used.
Dual trigger
The addition of low doses of HCG (1000–2500 IU) to GnRHa as a trigger was first suggested by Shapiro.[19] When giving GnRHa alone as a trigger, early pregnancy loss[20] and empty follicle syndrome[21] were reported in studies. The Shapiro group found that concomitant administration of GnRHa and HCG (1000–2500 iu) (depending on the BMI and OHSS risk factors) resulted in acceptable implantation, clinical pregnancy rates and ongoing pregnancy rateswithout the risk of OHSS in hyper responders. This was further supported by various studies.[22,23]
Dual trigger was tried in normal responders with improved oocyte yield[24]; besides, giving low-dose HCG with GnRHa allows one to do a fresh transfer with good implantation rates. It is recommended for poor responders with mature oocyte yield.[25]
The advantages of dual trigger are following:
GnRHa component causes release of both endogenous FSH and LH and increases the number of mature oocytes retrieved.
HCG supports corpus luteum function and supports implantation.
Endometrial receptivity increased by the GnRHa component.
Where there is no need to freeze all, fresh transfer can be done.
LH signal leads to high levels of epidermal growth factors (EGF) like amphiregulin, epiregulin, and beta cellulin that plays important role in cumulus expansion and oocytes maturation.[26,27] Dual trigger was tried in normal responders with good results.[28]
Double trigger:
The concept of a double trigger in GnRHa and HCG is given not together but 40 and 34 hours prior to oocyte retrieval, respectively.[29] This has been successfully tried in empty follicle syndrome, immature oocyte yield or poor oocyte yield.[30] More studies are needed for this trigger.
CONCLUSIONS
COH is a crucial component of assisted reproductive technologies like IUI and IVF. Triggers in COH play a pivotal role in maturation of the follicles in its final stages and optimizing the timing of egg retrieval, which is very important for the collection of maximum eggs and a good success rate.
One has to individualise the patients so that the appropriate trigger is given in each case. Kisspeptin seems to be a good option, but it needs more research.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Molecular mechanisms of ovulation and luteinization. Mol Cell Endocrinol. 1998;145:47-5.
- [CrossRef] [PubMed] [Google Scholar]
- Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147:1148-53.
- [CrossRef] [PubMed] [Google Scholar]
- Use of recombinant human chorionic gonadotropin in ovulation induction. Fertil Steril. 2003;79:1051-9.
- [CrossRef] [PubMed] [Google Scholar]
- Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol. 2005;234:75-9.
- [CrossRef] [PubMed] [Google Scholar]
- Disappearance of exogenously administered human chorionic gonadotropin. Fertil Steril. 1989;52:398-400.
- [CrossRef] [PubMed] [Google Scholar]
- “Triggering” of ovulation after infusion of synthetic luteinizing hormone releasing factor (LRF) Acta Obstet Gynecol Scand. 1973;52:269-72.
- [CrossRef] [PubMed] [Google Scholar]
- Hormonal dynamics at midcycle: a reevaluation. J Clin Endocrinol Metab. 1983;57:792-6.
- [CrossRef] [PubMed] [Google Scholar]
- Copenhagen GnRH Agonist Triggering Workshop Group. GnRH agonist for triggering of final oocyte maturation: time for a change of practice? Hum Reprod Update. 2011;17:510-24.
- [CrossRef] [PubMed] [Google Scholar]
- Follicle stimulating hormone alone supports follicle growth and oocyte development in gonadotrophin-releasing hormone antagonist-treated monkeys. Hum Reprod. 1995;10:1658-66.
- [CrossRef] [PubMed] [Google Scholar]
- Triggering with human chorionic gonadotropin or a gonadotropin-releasing hormone agonist in gonadotropin-releasing hormone antagonist-treated oocyte donor cycles: findings of a large retrospective cohort study. Fertil Steril. 2009;91:365-71.
- [CrossRef] [PubMed] [Google Scholar]
- 1,500 IU human chorionic gonadotropin administered at oocyte retrieval rescues the luteal phase when gonadotropin-releasing hormone agonist is used for ovulation induction: a prospective, randomized, controlled study. Fertil Steril. 2010;93:847-54.
- [CrossRef] [PubMed] [Google Scholar]
- The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomized controlled study. Fertil Steril. 2008;89(1):84-91.
- [CrossRef] [PubMed] [Google Scholar]
- Human recombinant luteinizing hormone is as effective as, but safer than, urinary human chorionic gonadotropin in inducing final follicular maturation and ovulation in in vitro fertilization procedures: results of a multicenter double-blind study. J Clin Endocrinol Metab. 2001;86:2607-18.
- [CrossRef] [Google Scholar]
- Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles. Cochrane Database Syst Rev. 2016;4:CD003719.
- [CrossRef] [Google Scholar]
- The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614-27.
- [CrossRef] [PubMed] [Google Scholar]
- Kisspeptin-54 triggers egg maturation in women undergoing in vitro fertilization. J Clin Invest. 2014;124:3667-77.
- [CrossRef] [PubMed] [Google Scholar]
- Design and synthesis of an Investigational Nonapeptide KISS1 Receptor (KISS1R) agonist, Ac-d-Tyr-Hydroxyproline (Hyp)-Asn-Thr-Phe-azaGly-Leu-Arg(Me)-Trp-NH2 (TAK-448), with highly potent testosterone-suppressive activity and excellent water solubility. J Med Chem. 2016;59(19):8804-11.
- [CrossRef] [PubMed] [Google Scholar]
- Gonadotropin-releasing hormone agonist combined with a reduced dose of human chorionic gonadotropin for final oocyte maturation in fresh autologous cycles of in vitro fertilization. Fertil Steril. 2008;90:231-3.
- [CrossRef] [PubMed] [Google Scholar]
- Gonadotropin-releasing hormone agonist combined with a reduced dose of human chorionic gonadotropin for final oocyte maturation in fresh autologous cycles of in vitro fertilization. Fertil Steril. 2008;90:231-3.
- [Google Scholar]
- GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study. Hum Reprod. 2005;20:1213-20.
- [CrossRef] [PubMed] [Google Scholar]
- Empty follicle syndrome after GnRHa triggering versus hCG triggering in COS. J Assist Reprod Genet. 2012;29:249-53.
- [CrossRef] [PubMed] [Google Scholar]
- Combined ovulation triggering with GnRH agonist and hCG in IVF patients. Gynecol Endocrinol. 2016;32(11):861-5.
- [CrossRef] [PubMed] [Google Scholar]
- Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist-assisted reproductive technology. Cochrane Database Syst Rev 2014:CD008046. doi: 10.1002/14651858. CD008046. pub4. PMID: 25358904. Cochrane analysed 17RCTs (No:1847)
- [CrossRef] [PubMed] [Google Scholar]
- Dual trigger improves the pregnancy rate in fresh in vitro fertilization (IVF) cycles compared with the human chorionic gonadotropin (hCG) trigger: a systematic review and meta-analysis of randomized trials. J Assist Reprod Genet. 2023;40:2063-77.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative study between single versus dual trigger for poor responders in GnRH-antagonist ICSI cycles: a randomized controlled study. Int J Gynaecol Obstet. 2021;152:395-400.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of FSH on the expression of receptors for oocyte-secreted factors and members of the EGF-like family during in vitro maturation in cattle. Reprod Fertil Dev. 2013;25:890-9. doi: 10.1071/ RD12125. PMID: 23021259
- [CrossRef] [PubMed] [Google Scholar]
- EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682-4.
- [CrossRef] [PubMed] [Google Scholar]
- Combined ovulation triggering with GnRH agonist and hCG in IVF patients. Gynecol Endocrinol. 2016;32:861-5.
- [Google Scholar]
- Dual trigger with gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves oocyte yield in normal responders on GnRH-antagonist cycles. JBRA Assist Reprod. 2022;26:28-32.
- [CrossRef] [PubMed] [Google Scholar]
- Empty follicle syndrome: successful treatment in a recurrent case and review of the literature. Hum Reprod. 2012;27:1357-67.
- [CrossRef] [PubMed] [Google Scholar]







