Translate this page into:
Sugar-sweetened beverage intake in relation to semen quality in infertile couples − a prospective observational study
Address for correspondence: Dr. Indrani Ghosh, Quarter type IV/38, Old Campus, SGPGIMS, Lucknow, 226014, Uttar Pradesh, India. E-mail: drindranighosh@yahoo.in
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aims:
This cross-sectional study was designed to evaluate effect of sugar-sweetened beverages (SSBs) on semen parameters in infertile couples.
Setting:
Men attending infertility clinic.
Design:
prospective, observational study.
Methods and material:
Participants were assessed the type and frequency of SSB intake in the past month by a previously validated beverage intake questionnaire (BEVQ-15) and provided one semen sample for analysis. The primary outcome were semen parameters; namely volume (in mL), total sperm count (in millions/ejaculate), sperm concentration (in million/mL), sperm motility% (PRM+NPM+IM), progressive motility% (PRM), vitality% and sperm morphology% (per 200 spermatozoa). The main independent variable was SSB intake.
Statistical analysis:
Multiple linear regression models were used to predict semen parameters based on SSB intake and potential confounders, compared across quartiles using Kruskal-Wallis test. Non-linearity was examined by fitting models with linear and quadratic terms.
Results:
385 men were included in the study. A significant decline in crude sperm motility% (P<0.001), progressive motility% (P<0.001), vitality% (P=0.017) and normal sperm morphology (P=0.006) with increasing SSB intake was found, along with a significant decline in volume and sperm concentration in the adjusted model (P<0.05). Significant decrease was noted in sperm motility and progressive motility percentages in both lean (BMI <25) and overweight and obese men (BMI ≥25) with increasing SSB intake. However, in presence of other potential confounders, SSBs lost its impact on semen parameters in the linear and quadratic fitted models, possibly due to interdependence of the confounders.
Conclusion:
Intake of SSBs negatively affect sperm parameters, except total sperm counts. But with other stronger confounders, its impact needs to further evaluated in larger populations of men.
Keywords
beverage intake questionnaire
sugar-sweetened beverage
semen parameters
INTRODUCTION
A prime cause of rising prevalence of infertility has been declining semen quality.
The ascendency of research on sugared drinks has been due to a growing concern on its detrimental effects on human fertility. Obesity and insulin resistance in the present day have contributed to reduced sperm production by altering the hypothalamic–pituitary–gonadal axis.[1] Sugar-sweetened beverages (SSBs) have themselves been implicated in causing obesity and insulin resistance.[2] Semen quality in Indian men have undergone a temporal decline,[3] similar to reports from around the world.[4,5] This study was undertaken to evaluate the effect of SSBs on semen parameters in Indians, attending an infertility clinic.
MATERIAL AND METHODS
This prospective observational study was conducted in men aged 18 to 50 years attending infertility clinic. A sample size of 385 was calculated, considering proportion of sub-fertile males as 50%[6,7,8] and a 95% confidence interval. Couple anticipating use of their own gametes for infertility treatment and men with no history of vasectomy and ready to undergo at least one semen analysis were included. Men with semen analysis produced prior to dietary assessment, or with missing semen parameter data, with history of reproductive organ disorder or on medications to improve semen parameters were excluded. After proper informed consent being taken, participants were provided questionnaire based on beverage intake in the past 1 month. On entry upon the study, they underwent physical examination and provided semen sample for analysis. Men were instructed to abstain from ejaculation for at least 48 hours before sample collection (men not following this were identified but not excluded), and participants were asked to report abstinence period at the time of sample collection. Semen analysis was done according to WHO 2010 criteria. Ejaculate volume was estimated by specimen weight, assuming a semen density of 1.0 g/mL. Sperm concentration was estimated using Makler chamber (Irvine Scientific 2511 Daimler, St. Santa Ana, CA, USA). Sperm motility was classified as progressive (WHO class A + B) and total (WHO class A + B + C). Morphology was assessed using strict criteria.[9] Total sperm count and sperms with normal morphology and vitality were counted.
SSB intake was assessed using a previously validated 15-item beverage intake questionnaire[10] (food frequency questionnaire, FFQ). Responses to the frequency of intake, ‘how often’ ranged from ‘never or less than 1 time per week’ to more than 3+ times per day’; ‘how much’ ranged from ‘less than 3/4th of a cup’ to ‘more than 2½ cup’. Nutrient intake estimates were derived from the nutrient database of US Department of Agriculture (USDA). Other variables examined as potential confounders included age, BMI, physical activity (moderate exercise < 10.5 h/week or vigorous exercise ≥ 10.5 h/week), educational level, smoker, tobacco intake in any other form like chewing, abstinence time in hours, total caffeine intake (g/day) and total alcohol intake (g/day), along with reproductive history of genital disease, varicocele/hydrocele/cryptorchidism.
Smokers were divided in the following groups: NEVER − those who never smoked, PREVIOUS − those who quit smoking for more than 6 months, OCCASSIONAL − one or less than one cigarette smoked per week, PRESENT − more than one cigarette smoked per week. Similar groups were created for men who chewed tobacco (cigarettes replaced by number of times tobacco chewed).
Men were classified in quartiles of SSB intake. Variation in semen parameters across increasing quartiles of SSB intake was compared by Kruskal–Wallis test. Non-linearity was examined by fitting models with linear and quadratic terms. Multiple linear regression models were used to predict the semen parameters based on SSB intake and potential confounders compared across the quartiles using Kruskal–Wallis test.
The statistical software SPSS version 20 (IBM) has been used for the analysis. An alpha level of 5%, that is, any P value less than 0.05, was considered significant.
RESULTS
Three hundred eighty-five men underwent dietary assessment and semen analysis. The participants were divided into quartiles of SSB intake (kcal/day) as follows: Q1: ˂140.4, Q2: 140.4–280, Q3: 280.1–470, Q4: >470. The number of men in each quartile was comparable; 60.50% of the participants were in the age group of 31 to 40 years, 51.20% of men had normal BMI and 83.6% of participants were educated up to or beyond class XII. The mean (standard deviation) age of participants was 36.85 (5.93) years, sleep was 7.11 (1.49) hours/day, exercise was 2.77 (3.82) hours/week, abstinence was 5.84 (5.61) days, alcohol intake was 3.41 (10.27) g/day and caffeine intake was 166.36 (112.90) mg/day. The mean (standard deviation, SD) SSB intake and total beverage intake in the study population was 345.17 (266.31) and 447.90 kcal/d, respectively. SSB intake was 71.55% of beverage intake; 51.70% and 53.80% of the participants were non-smokers and non-tobacco chewers, respectively, and the difference across the quartiles was not significant for smoking (P value = 0.461) and tobacco chewing (P value = 0.457).
Men who consumed higher quantity of SSBs had significantly higher intake of caffeine, sweetened juice/beverage/drink, soft drinks, sweetened tea, tea/coffee with cream and/or sugar and tea/coffee black (P < 0.05) [Table 1]; 69.72% of SSB intake was made of tea or coffee with cream and/sugar and 6.96% of total SSB intake was made of regular soft drinks.

The difference across the quartiles of SSB intake for sperm motility% and progressive motility% were highly significant (P < 0.001) and that of sperm vitality and normal sperm morphology were significant (P = 0.017 and P = 0.006, respectively) [Table 2].

On analysing semen parameters, after adjustment for potential confounders using linear and quadratic equations with SSB intake, it was seen that in the presence of other confounders, SSBs lost its impact on sperm parameters, probably due to multi-collinearity, as the independent variables can be highly interdependent [Figure 1a]–g].

- Effect on semen parameters after adjustment for potential confounders using linear and quadratic equations with SSB intake. (a) SSB intake in relation with total semen volume (in mL), modelled with linear and quadratic fit. *Solid lines represent estimate of linear regression model; dashed lines represent 95% confidence limits of the estimate. Models are adjusted for age, BMI, sleep duration, exercise, smoking, tobacco chewing, alcohol intake, caffeine intake and total SSB intake. (b) SSB intake in relation with total sperm count (in million/ejaculate). (c) SSB intake in relation with sperm concentration (in million/mL). (d) SSB intake in relation with sperm motility%. (e) SSB intake in relation with progressive sperm motility%. (f) SSB intake in relation with sperm vitality%. (g) SSB intake in relation to normal sperm morphology (per 200 spermatozoa). BMI, body mass index; SSB, sugar-sweetened beverage.
Results of multiple linear regression on sperm parameters show that the only significant predictors of volume in millilitre were exercise which had a positive impact, and alcohol intake which had a negative impact; that for total sperm count was exercise which had a positive impact and the variable affecting sperm vitality was age which had negative impact, that is as age rose, vitality decreased. The predicted values of all sperm parameters, except total sperm count (in million/ejaculate), obtained from the multiple linear regression model [Table 3], after adjusting for confounders like age, BMI, sleep duration, exercise, smoking, tobacco chewing and alcohol consumption and total SSB intake, vary significantly across the quartiles of SSB intake. Except for sperm concentration which was significantly different across the quartiles (P = 0.034), differences in semen volume, sperm motility%, progressive sperm motility, sperm vitality and normal sperm morphology were highly significant (P < 0.001) across the quartiles.
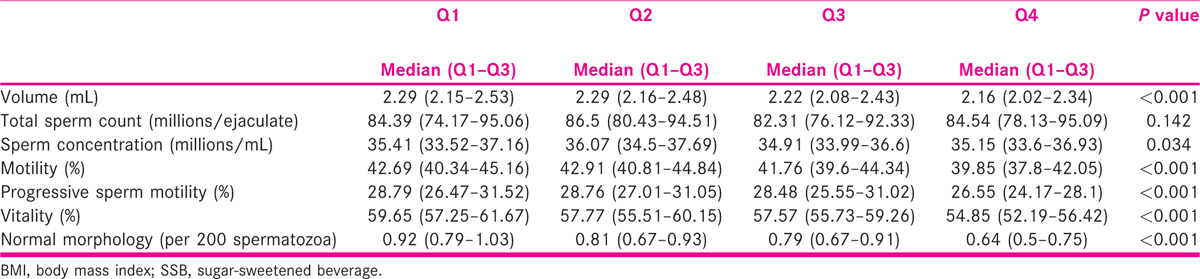
The decline in mean semen volume from Q1 to Q4 was by 5.6%, an increase in mean total sperm count by 3.7%, no change in mean sperm concentration, a decline in sperm motility by 7.6%, a decline in mean progressive sperm motility by 10.7% and a decline in mean sperm vitality and mean normal sperm morphology by 8.6% and 32.5%, respectively [Table 4].

BMI had modified the relation between SSB intake and sperm parameters, only in the context of sperm motility (along with progressive motility), other parameters remaining unaffected. Significant decrease was noted in sperm motility and progressive motility percentages in both lean (BMI < 25) and overweight and obese men (BMI ≥ 25) across the quartiles of SSB intake [Tables 5 and 6].


DISCUSSION
The present study, to the best of our knowledge, is the only study in the Indian population regarding the effect of specific SSBs on semen parameters. Our study is also important in validating the deleterious effects of SSB intake on semen quality in men attending infertility clinics, especially in India, where SSBs form a part of regular diet and the types of SSBs are also different than the Western countries. Smoking as a confounder was not found to be significantly different across the quartiles of SSB intake in our study (P = 0.461), similar to the previous studies.[11,12,13,14] Tobacco chewing has also not been found to be significantly different across the quartiles of SSB intake in our study (P = 0.457). The deleterious effects of tobacco chewing on semen volume, sperm concentration, sperm motility and viability was well documented in some Indian studies.[15,16] However, none of the relevant previous studies considered it as a potential confounder on the effect of SSB intake on semen quality, primarily because the habit is largely prevalent in the Indian subcontinent.
Although the univariate analysis did not reveal any significant relation between alcohol and increasing quartiles of SSB intake (P = 0.279), similar to findings of Chiu et al.[11] (P = 0.38), the multivariate analysis revealed a negative impact of alcohol intake on semen volume in our study (P = 0.020). Alcohol intake was not related to any other semen parameter in our study. Alcohol intake has been associated with increased β-endorphin levels which might be implicated in testicular damage, inducing apoptosis of sperms[17] and fertilisation failure in humans.[18]
None of the semen parameters were affected by caffeine intake in the multivariate model in our study, the mean caffeine intake being 166.36 (±112.90) mg/day, similar to the median caffeine intake of Karmon et al.[19] (161 mg, as against 159 mg in our study). Caffeine consumption of ≤800 mg/day and cola consumption of ≤14 0.5-L bottles/week was unassociated with poor semen quality,[20] which was however apparent after cola consumption of 1 L/day, which was attributed to the constituents of cola rather than caffeine itself. A recent systematic review[21] reported no effect on semen quality by caffeine in coffee, tea and cocoa drinks in most studies.[19,22,23,24] However, some studies reported a negative association of cola and caffeinated drinks with sperm count, concentration and volume.[20,25] Caffeine consumption was associated with increased testosterone levels[22] and seemed to be linked with aneuploidy and DNA breaks.[26] These findings reinforce our understanding that standardised DNA fragmentation tests are required to elucidate poor sperm function, fertilisation rates and embryo quality in apparently normozoospermic men undergoing analysis of routine semen parameters.
Tea/coffee with cream and/or sugar was significantly increased in men in higher quartiles of SSB intakes than in the lowest quartile in the univariate analyses (P < 0.001). It also was the beverage with the highest intake in the participants (69.72% of mean beverage intake). The mean intake of regular soft drinks in our study was 24.03 ± 49.02 kcal/day and that of diet soft drink was very low (0.17 ± 2.84). However, the intake of regular soft drink was significantly different when the higher quartiles of SSB intake was compared to the lowest quartile (P < 0.001). This difference was not observed in relation to diet soft drinks and energy drinks. Men whose cola intake was >1 L/day had significantly reduced total sperm counts and sperm concentration than non-consumers; this finding was not explained by quantity of caffeine intake.[20] Two former studies[11,27] had both calculated the servings of SSB/day which would provide different energy intake for different SSB, but in our study, we had calculated the exact kcal/day contributed by a drink, and thus, our calculation seems to be more precise; 45% of the SSB intake was by non-carbonated drinks in the study by Chiu et al.,[11] whereas in our study, 91.56% of SSB intake was by non-carbonated drinks. Also, the level of physical activity differs in between the studies, which might explain the differences in associations with soft drinks. Leached phalates and bisphenol A from plastic containers of sugared colas can also explain the deterioration of semen quality in cola drinkers, apart from the effect of the caffeine content.[14,28]
A precious Indian article,[3] studying the semen parameters in Indian men, comparing fertile and infertile participants, had provided the reference semen parameters for Indian men. Our mean semen indices were remarkably similar to those of the infertile Indian men in the study, thereby further strengthening the reference values relevant in our population. Our results were consistent with those of animal studies which had demonstrated 25.3% fewer offspring per population in fructose/glucose-fed male mice than controls.[29] Furthermore, epididymal sperm motility, sperm concentration and viability were reduced significantly (P < 0.05) in sucrose-fed Sprague–Dawley rats with associated reduced levels of testosterone and increased levels of corticosterone.[30] A higher level of lipid peroxidation in rat testes, fed with high sucrose, and subsequent induction of superoxide free radicles was postulated to impair sperm function.[30] Our results were also coincident with studies on effect of high sugary drinks on male fertility in humans. Lower total and progressive sperm motility in higher quartiles of SSB intake in young healthy men were independent of confounders but primarily linked to lean men,[11] attributed to obesity which had deleterious effects on sperm motility and was outweighed by its modest association with SSBs.
Sperm concentration and normal sperm morphology were lower in the highest quartiles of intake of ‘Western diet’ and ‘High sweet snacks and sugar sweetened drinks’ in the study by Chiu et al.,[11] the first of its kind in an Asian population which had a predominantly higher carbohydrate intake. Dietary patterns and its effect on male fertility were also studied by several authors in different parts of the world. A positive correlation was noted between serum testosterone levels and sperm concentration with ‘Prudent diet’ as compared to ‘Western diet’ rich in processed meat, high-fat dairy, refined grains, snacks, high energy drinks and sweets.[14] Prudent diet intake, higher content of fish, chicken, tomatoes, fruit, cruciferous and leafy green vegetables and whole grains revealed increased sperm concentration, increased serum testosterone levels and significantly lesser sperms with DNA damage, along with lesser occurrence of disomy of chromosomes XX and 21.[14] Similarly, a strong adherence to Western diet had a positive correlation with abnormal progressive motility in the crude model, whereas in the adjusted model, a similar adherence was related to increased risk of abnormal sperm morphology, abnormal total sperm count and progressive motility.[31] A recent systematic review concluded that adherence to a healthy diet pattern does improve male fertility.[32]
In both the groups of men with BMI <25 and ≥25, total motility% and progressive motility% significantly varied across the quartiles; other parameters were unaffected significantly. Chiu et al.[11] demonstrated this effect only on lean men[11] whereas Sermonade et al.[1] found sperm concentration and count strongly related to obese men. Obesity increases insulin resistance which negatively influences quality of semen by increased oxidative stress.[1,33]Our study showed that SSB is a good predictor of semen parameters individually, but when other confounding factors like age, BMI, exercise, smoking, tobacco chewing and sleep are also taken in consideration in the linear and quadratic equations with SSB, it loses its impact, probably due to interdependence of the variables. This was different from the study by Chiu et al.[11] which showed that SSB intake significantly decreases sperm motility, fecundability after adjustment for confounders in the linear and quadratic fit models.[11] Possible explanations could be the following:
Relatively smaller study population.
Type of SSB intake in the studied population was different from most of the other studies, we had more consumption of sweetened tea and tea/coffee with cream and or/sugar, than soft drinks and sugared colas.
Most studies[20,21] showing intake of caffeine negatively affecting sperm parameters have attributed it to ‘sugared colas’ and not intake from tea/coffee or chocolate drinks. Tea itself has shown an increase in female fecundity[34] and intake of green tea has been shown to reduce reactive oxygen species (ROS) production therefore improving classical sperm parameters.[35]
Role of stress and diet in male infertility not evaluated.
The study only included questions on specific beverages, and not food habits or dietary patterns as a whole, whose cumulative effect on sperm parameters could have explained the associations in a better manner.
The FFQ used was prepared and validated in Western countries where the types of beverage intake are quite different than those in India. However, we lack a standardised beverage intake questionnaire and developing one could be helpful for further research work in this subcontinent.
The population of men attending infertility clinics are different from the healthy young men studied by some authors. Their cause of infertility could be multiple, with inter-related factors. Also, diagnosis of a disease could lead to changed or differential reporting of diet.
Strengths of our study were that participants were blinded to the results of the study, thus reducing possibility of reverse causation; use of a previously validated beverage intake questionnaire, covering a large range of beverages; possibly the first kind of study in the Indian population; assessment of tobacco chewing in the participants as a confounder prevalent in the population studied and a detailed assessment of various lifestyle factors, along with detailed reproductive history and physical examination, allowed for adjustment of a large number of potential confounders in the study population.
Our study had some limitations. The cross-sectional design of the study, interfering with our ability to study causality; the intake of beverages in the past month might not have been the same as that of the previous 3 months, which is the duration of spermatogenesis prior to producing the ejaculate for analysis; men attending infertility clinics might have differentially reported the intake of certain beverages, alcohol, smoking or tobacco chewing, knowing its harmful effects on general health, thus contributing to recall bias; assessment of a single semen sample from each participant might have induced errors in assessing the quality, and thereby association with SSB intake, and the use of only a beverage intake questionnaire, instead of assessing the entire food choice of participants might have induced residual confounding by other dietary factors.
CONCLUSION
In conclusion, we found a statistically significant decline in crude semen parameters of sperm motility%, progressive motility%, sperm vitality and normal sperm morphology with increasing SSB intake which correlated with the adjusted model of semen quality parameters in the multivariate analysis, along with a significant decline in semen volume and sperm concentration in the adjusted model. But in presence of other stronger confounders, the impact of SSBs on semen quality needs to further evaluated in larger populations of men attending infertility clinics and to determine how much of this translates to actual male fertility.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19:221-31.
- [Google Scholar]
- Consuming fructose-sweetened, not-glucose sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322-34.
- [Google Scholar]
- Decline in seminal quality in Indian men over the last 37 years. Reprod Biol Endocrinol. 2018;16:103.
- [Google Scholar]
- Male factor infertility: evaluation and management. Med Clin North Am. 2004;88:367-85.
- [Google Scholar]
- Men’s health − male factor infertility. University of Utah Health Sciences Centre; 2003. Archived from the original on July 4, 2007.
- The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5:586-92.
- [Google Scholar]
- Development of a brief questionnaire to assess habitual beverage intake (BEVQ-15): sugar-sweetened beverage and total beverage energy intake. J Acad Nutr Diet. 2012;112:840-9.
- [Google Scholar]
- Sugar-sweetened beverage intake in relation to semen quality and reproductive hormone levels in young men. Hum Reprod. 2014;29:1575-84.
- [Google Scholar]
- Dairy food intake in relation to semen quality and reproductive hormone levels among physically active young men. Hum Reprod. 2013;28:2265-75.
- [Google Scholar]
- Dietary patterns and their relationship with semen quality. Am J Men’s Health. 2018;12:575-83.
- [Google Scholar]
- Effect of tobacco chewing on semen parameters. Int J Med Sci Pub Health. 2016;5:1139-42.
- [Google Scholar]
- Relationship between semen quality and tobacco chewing in men undergoing infertility evaluation. Fertil Steril. 2005;84:649-53.
- [Google Scholar]
- Effect of alcohol consumption on in vitro fertilization. Obstet Gynecol. 2011;117:136-42.
- [Google Scholar]
- Male caffeine and alcohol intake in relation to semen parameters and in vitro fertilization outcomes among fertility patients. Andrology. 2017;5:354-61.
- [Google Scholar]
- Caffeine intake and semen quality in a population of 2,554 young Danish men. Am J Epidemiol. 2010;171:883-91.
- [Google Scholar]
- Dietary habits and semen parameters: a systematic narrative review. Andrology. 2018;6:104-16.
- [Google Scholar]
- Semen quality according to prenatal coffee and present caffeine exposure: two decades of follow-up of a pregnancy cohort. Hum Reprod. 2008;23:2799-805.
- [Google Scholar]
- Bulky DNA adducts in human sperm: relationship with fertility, semen quality, smoking, and environmental factors. Mutat Res. 2003;537:53-65.
- [Google Scholar]
- Sperm DNA damage − the effect of stress and everyday life factors. Int J Impot Res. 2016;28:148-54.
- [Google Scholar]
- Caffeine and caffeinated beverage consumption and fecundability in a preconception cohort. Repr Toxicol. 2016;62:39-45.
- [Google Scholar]
- Use of fluorescence in situ hybridization (FISH) to assess effects of smoking, caffeine, and alcohol on aneuploidy load in sperm of healthy men. Environ Mol Mutagen. 1997;30:175-83.
- [Google Scholar]
- Intake of sugar-sweetened beverages and fecundability in a North American preconception cohort. Epidemiology. 2018;29:369-78.
- [Google Scholar]
- Sperm counts may have declined in young university students in Southern Spain. Andrology. 2013;1:408-13.
- [Google Scholar]
- Human-relevant levels of added sugar consumption increase female mortality and lower male fitness in mice. Nat Commun. 2013;4:2245.
- [Google Scholar]
- Consumption of high sucrose and/or high salt diet alters sperm function in male Sprague-Dawley rats. Egypt J Basic Appl Sci. 2016;3:194-201.
- [Google Scholar]
- Dietary patterns and poor semen quality risk in men: a cross-sectional study. Nutrients. 2018;10:1162.
- [Google Scholar]
- Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23:371-389.
- [Google Scholar]
- Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. 2009;32:1302-7.
- [Google Scholar]
- Caffeinated beverage and soda consumption and time to pregnancy. Epidemiology. 2012;23:393-401.
- [Google Scholar]
- Therapeutic role of green tea polyphenolsin improving fertility: a review. Nutrients. 2018;10:834.
- [Google Scholar]







